FRS2α-mediated FGF signals suppress premature differentiation of cardiac stem cells through regulating autophagy activity
- PMID: 22207710
- PMCID: PMC3677753
- DOI: 10.1161/CIRCRESAHA.111.255950
FRS2α-mediated FGF signals suppress premature differentiation of cardiac stem cells through regulating autophagy activity
Abstract
Rationale: Although the fibroblast growth factor (FGF) signaling axis plays important roles in heart development, the molecular mechanism by which the FGF regulates cardiogenesis is not fully understood.
Objective: To investigate the mechanism by which FGF signaling regulates cardiac progenitor cell differentiation.
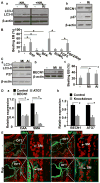
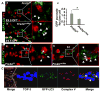
Methods and results: Using mice with tissue-specific ablation of FGF receptors and FGF receptor substrate 2α (Frs2α) in heart progenitor cells, we demonstrate that disruption of FGF signaling leads to premature differentiation of cardiac progenitor cells in mice. Using embryoid body cultures of mouse embryonic stem cells, we reveal that FGF signaling promotes mesoderm differentiation in embryonic stem cells but inhibits cardiomyocyte differentiation of the mesoderm cells at later stages. Furthermore, we also report that inhibiting FRS2α-mediated signals increases autophagy and that activating autophagy promotes myocardial differentiation and vice versa.
Conclusions: The results indicate that the FGF/FRS2α-mediated signals prevent premature differentiation of heart progenitor cells through suppressing autophagy. The findings provide the first evidence that autophagy plays a role in heart progenitor differentiation.
Figures






Comment in
-
Autophagy in myocardial differentiation and cardiac development.Circ Res. 2012 Feb 17;110(4):524-5. doi: 10.1161/CIRCRESAHA.112.265157. Circ Res. 2012. PMID: 22343554 No abstract available.
Similar articles
-
The fibroblast growth factor signaling axis controls cardiac stem cell differentiation through regulating autophagy.Autophagy. 2012 Apr;8(4):690-1. doi: 10.4161/auto.19290. Epub 2012 Apr 1. Autophagy. 2012. PMID: 22302007 Free PMC article.
-
Frs2alpha-deficiency in cardiac progenitors disrupts a subset of FGF signals required for outflow tract morphogenesis.Development. 2008 Nov;135(21):3611-22. doi: 10.1242/dev.025361. Epub 2008 Oct 2. Development. 2008. PMID: 18832393 Free PMC article.
-
BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors.Development. 2010 Sep;137(18):2989-3000. doi: 10.1242/dev.051649. Epub 2010 Aug 11. Development. 2010. PMID: 20702560
-
Second heart field cardiac progenitor cells in the early mouse embryo.Biochim Biophys Acta. 2013 Apr;1833(4):795-8. doi: 10.1016/j.bbamcr.2012.10.003. Epub 2012 Oct 7. Biochim Biophys Acta. 2013. PMID: 23051926 Review.
-
Control of stemness by fibroblast growth factor signaling in stem cells and cancer stem cells.Curr Stem Cell Res Ther. 2009 Jan;4(1):9-15. doi: 10.2174/157488809787169048. Curr Stem Cell Res Ther. 2009. PMID: 19149625 Review.
Cited by
-
FGF7/KGF regulates autophagy in keratinocytes: A novel dual role in the induction of both assembly and turnover of autophagosomes.Autophagy. 2014 May;10(5):803-21. doi: 10.4161/auto.28145. Epub 2014 Feb 26. Autophagy. 2014. PMID: 24577098 Free PMC article.
-
Autophagy-Related Protein MAP1LC3C Plays a Crucial Role in Odontogenic Differentiation of Human Dental Pulp Cells.Tissue Eng Regen Med. 2021 Apr;18(2):265-277. doi: 10.1007/s13770-020-00310-3. Epub 2020 Nov 24. Tissue Eng Regen Med. 2021. PMID: 33230801 Free PMC article.
-
Understanding the molecular mechanisms and role of autophagy in obesity.Mol Biol Rep. 2021 Mar;48(3):2881-2895. doi: 10.1007/s11033-021-06298-w. Epub 2021 Apr 2. Mol Biol Rep. 2021. PMID: 33797660 Review.
-
Autophagy in cardiovascular biology.J Clin Invest. 2015 Jan;125(1):55-64. doi: 10.1172/JCI73943. Epub 2015 Jan 2. J Clin Invest. 2015. PMID: 25654551 Free PMC article. Review.
-
Loss of Fgfr1 in chondrocytes inhibits osteoarthritis by promoting autophagic activity in temporomandibular joint.J Biol Chem. 2018 Jun 8;293(23):8761-8774. doi: 10.1074/jbc.RA118.002293. Epub 2018 Apr 24. J Biol Chem. 2018. PMID: 29691281 Free PMC article.
References
-
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. - PubMed
-
- Srivastava D. Making or breaking the heart: From lineage determination to morphogenesis. Cell. 2006;126:1037–1048. - PubMed
-
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from fgf10-expressing cells in pharyngeal mesoderm. Developmental cell. 2001;1:435–440. - PubMed
-
- McKeehan WL, Wang F, Luo Y. The fibroblast growth factor (fgf) signaling complex. Handbook of cell signaling. New York: Academic/Elsevier Press; 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

