Peroxisome proliferator-activated receptor-γ activation induces 11β-hydroxysteroid dehydrogenase type 1 activity in human alternative macrophages
- PMID: 22207732
- PMCID: PMC3428270
- DOI: 10.1161/ATVBAHA.111.241364
Peroxisome proliferator-activated receptor-γ activation induces 11β-hydroxysteroid dehydrogenase type 1 activity in human alternative macrophages
Abstract
Objective: 11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1) catalyzes the intracellular reduction of inactive cortisone to active cortisol, the natural ligand activating the glucocorticoid receptor (GR). Peroxisome proliferator- activated receptor-γ (PPARγ) is a nuclear receptor controlling inflammation, lipid metabolism, and the macrophage polarization state. In this study, we investigated the impact of macrophage polarization on the expression and activity of 11β-HSD1 and the role of PPARγ therein.
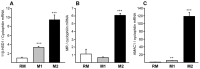
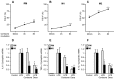
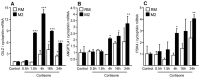
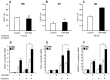
Methods and results: 11β-HSD1 gene expression is higher in proinflammatory M1 and anti-inflammatory M2 macrophages than in resting macrophages, whereas its activity is highest in M2 macrophages. Interestingly, PPARγ activation induces 11β-HSD1 enzyme activity in M2 macrophages but not in resting macrophages or M1 macrophages. Consequently, human M2 macrophages displayed enhanced responsiveness to the 11β-HSD1 substrate cortisone, an effect amplified by PPARγ induction of 11β-HSD1 activity, as illustrated by an increased expression of GR target genes.
Conclusion: Our data identify a positive cross-talk between PPARγ and GR in human M2 macrophages via the induction of 11β-HSD1 expression and activity.
Figures


Similar articles
-
Effects of peroxisome proliferator-activated receptor-alpha and -gamma agonists on 11beta-hydroxysteroid dehydrogenase type 1 in subcutaneous adipose tissue in men.J Clin Endocrinol Metab. 2007 May;92(5):1848-56. doi: 10.1210/jc.2006-2713. Epub 2007 Feb 27. J Clin Endocrinol Metab. 2007. PMID: 17327378 Clinical Trial.
-
Is the metabolic syndrome an intracellular Cushing state? Effects of multiple humoral factors on the transcriptional activity of the hepatic glucocorticoid-activating enzyme (11beta-hydroxysteroid dehydrogenase type 1) gene.Mol Cell Endocrinol. 2008 Mar 26;285(1-2):10-8. doi: 10.1016/j.mce.2008.01.012. Epub 2008 Feb 2. Mol Cell Endocrinol. 2008. PMID: 18313835
-
Role of glucocorticoid receptor and CCAAT/enhancer-binding protein alpha in the feed-forward induction of 11beta-hydroxysteroid dehydrogenase type 1 expression by cortisol in human amnion fibroblasts.J Endocrinol. 2007 Nov;195(2):241-53. doi: 10.1677/JOE-07-0303. J Endocrinol. 2007. PMID: 17951535
-
The metabolism of steroids, toxins and drugs by 11β-hydroxysteroid dehydrogenase 1.Toxicology. 2012 Feb 6;292(1):1-12. doi: 10.1016/j.tox.2011.11.012. Epub 2011 Nov 28. Toxicology. 2012. PMID: 22154985 Review.
-
11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action.J Endocrinol. 2005 Aug;186(2):251-71. doi: 10.1677/joe.1.06019. J Endocrinol. 2005. PMID: 16079253 Review.
Cited by
-
Pioglitazone in adult rats reverses immediate postnatal overfeeding-induced metabolic, hormonal, and inflammatory alterations.Endocrine. 2015 Dec;50(3):608-19. doi: 10.1007/s12020-015-0657-z. Epub 2015 Jun 18. Endocrine. 2015. PMID: 26084260
-
11β-Hydroxysteroid Dehydrogenase Type 1 Is Expressed in Neutrophils and Restrains an Inflammatory Response in Male Mice.Endocrinology. 2016 Jul;157(7):2928-36. doi: 10.1210/en.2016-1118. Epub 2016 May 4. Endocrinology. 2016. PMID: 27145012 Free PMC article.
-
The effect of pioglitazone on aldosterone and cortisol production in HAC15 human adrenocortical carcinoma cells.Mol Cell Endocrinol. 2014 Aug 25;394(1-2):119-28. doi: 10.1016/j.mce.2014.07.007. Epub 2014 Jul 17. Mol Cell Endocrinol. 2014. PMID: 25038520 Free PMC article.
-
MicroRNA-130b attenuates dexamethasone-induced increase of lipid accumulation in porcine preadipocytes by suppressing PPAR-γ expression.Oncotarget. 2017 Sep 27;8(50):87928-87943. doi: 10.18632/oncotarget.21318. eCollection 2017 Oct 20. Oncotarget. 2017. PMID: 29152131 Free PMC article.
-
Peroxisome Proliferator-Activated Receptor γ Induces the Expression of Tissue Factor Pathway Inhibitor-1 (TFPI-1) in Human Macrophages.PPAR Res. 2016;2016:2756781. doi: 10.1155/2016/2756781. Epub 2016 Dec 27. PPAR Res. 2016. PMID: 28115923 Free PMC article.
References
-
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. - PubMed
-
- Chapman KE, Coutinho A, Gray M, Gilmour JS, Savill JS, Seckl JR. Local amplification of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1 and its role in the inflammatory response. Ann N Y Acad Sci. 2006;1088:265–273. - PubMed
-
- Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. - PubMed
-
- Ricketts ML, Verhaeg JM, Bujalska I, Howie AJ, Rainey WE, Stewart PM. Immunohistochemical localization of type 1 11beta-hydroxysteroid dehydrogenase in human tissues. J Clin Endocrinol Metab. 1998;83:1325–1335. - PubMed

