The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis
- PMID: 22207737
- PMCID: PMC3286349
- DOI: 10.1182/blood-2011-06-360685
The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis
Abstract
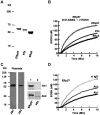
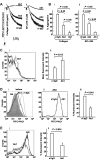
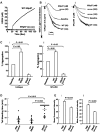
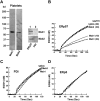
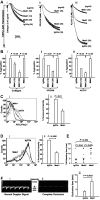
A close homologue to protein disulfide isomerase (PDI) called ERp57 forms disulfide bonds in glycoproteins in the endoplasmic reticulum and is expressed on the platelet surface. We generated 2 rabbit Abs to ERp57. One Ab strongly inhibited ERp57 in a functional assay and strongly inhibited platelet aggregation. There was minimal cross-reactivity of this Ab with PDI by Western blot or in the functional assay. This Ab substantially inhibited activation of the αIIbβ3 fibrinogen receptor and P-selectin expression. Furthermore, adding ERp57 to platelets potentiated aggregation. In contrast, adding a catalytically inactive ERp57 inhibited platelet aggregation. When infused into mice the inactive ERp57 prolonged the tail bleeding times. We generated 2 IgG2a mAbs that reacted with ERp57 by immunoblot. One of these Abs inhibited both ERp57 activity and platelet aggregation. The other Ab did not inhibit ERp57 activity or platelet aggregation. The inhibitory Ab inhibited activation of αIIbβ3 and P-selectin expression, prolonged tail bleeding times, and inhibited FeCl(3)-induced thrombosis in mice. Finally, we found that a commonly used mAb to PDI also inhibited ERp57 activity. We conclude that a glycoprotein-specific member of the PDI family, ERp57, is required for platelet aggregation, hemostasis, and thrombosis.
Figures
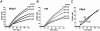
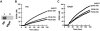
Similar articles
-
Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the αIIbβ3 integrin.Blood. 2013 Nov 21;122(22):3642-50. doi: 10.1182/blood-2013-06-506691. Epub 2013 Sep 12. Blood. 2013. PMID: 24030382 Free PMC article.
-
Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice.Blood. 2013 Aug 8;122(6):1052-61. doi: 10.1182/blood-2013-03-492504. Epub 2013 Jun 20. Blood. 2013. PMID: 23788140 Free PMC article.
-
Multiple protein disulfide isomerases support thrombosis.Curr Opin Hematol. 2018 Sep;25(5):395-402. doi: 10.1097/MOH.0000000000000449. Curr Opin Hematol. 2018. PMID: 29994898 Free PMC article. Review.
-
A novel role for endoplasmic reticulum protein 46 (ERp46) in platelet function and arterial thrombosis in mice.Blood. 2022 Mar 31;139(13):2050-2065. doi: 10.1182/blood.2021012055. Blood. 2022. PMID: 34752599 Free PMC article.
-
Thiol isomerases in thrombus formation.Circ Res. 2014 Mar 28;114(7):1162-73. doi: 10.1161/CIRCRESAHA.114.301808. Circ Res. 2014. PMID: 24677236 Free PMC article. Review.
Cited by
-
Regulatory role of thiol isomerases in thrombus formation.Expert Rev Hematol. 2018 May;11(5):437-448. doi: 10.1080/17474086.2018.1452612. Epub 2018 Mar 28. Expert Rev Hematol. 2018. PMID: 29542339 Free PMC article. Review.
-
The gut microbiota-bile acid-TGR5 axis orchestrates platelet activation and atherothrombosis.Nat Cardiovasc Res. 2025 May;4(5):584-601. doi: 10.1038/s44161-025-00637-x. Epub 2025 Apr 11. Nat Cardiovasc Res. 2025. PMID: 40217125
-
Identification and characterisation of the RalA-ERp57 interaction: evidence for GDI activity of ERp57.PLoS One. 2012;7(11):e50879. doi: 10.1371/journal.pone.0050879. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226417 Free PMC article.
-
Tyro3, Axl, and Mertk receptors differentially participate in platelet activation and thrombus formation.Cell Commun Signal. 2018 Dec 12;16(1):98. doi: 10.1186/s12964-018-0308-0. Cell Commun Signal. 2018. PMID: 30541554 Free PMC article.
-
Protein disulfide isomerase regulation by nitric oxide maintains vascular quiescence and controls thrombus formation.J Thromb Haemost. 2018 Nov;16(11):2322-2335. doi: 10.1111/jth.14291. Epub 2018 Oct 12. J Thromb Haemost. 2018. PMID: 30207066 Free PMC article.
References
-
- Goldberger RF, Epstein CJ, Anfinsen CB. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem. 1963;238:628–635. - PubMed
-
- Venetianer P, Straub FB. The enzymic reactivation of reduced ribonuclease. Biochim Biophys Acta. 1963;67:166–168. - PubMed
-
- Essex DW, Li M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br J Haematol. 1999;104(3):448–454. - PubMed
-
- Essex DW. Redox control of platelet function. Antioxid Redox Signal. 2009;11(5):1191–1225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

