The ubiquitin regulatory X (UBX) domain-containing protein TUG regulates the p97 ATPase and resides at the endoplasmic reticulum-golgi intermediate compartment
- PMID: 22207755
- PMCID: PMC3307297
- DOI: 10.1074/jbc.M111.284232
The ubiquitin regulatory X (UBX) domain-containing protein TUG regulates the p97 ATPase and resides at the endoplasmic reticulum-golgi intermediate compartment
Abstract
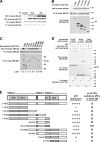
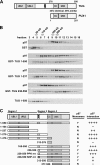
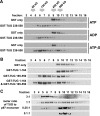
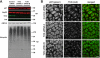
p97/VCP is a hexameric ATPase that is coupled to diverse cellular processes, such as membrane fusion and proteolysis. How p97 activity is regulated is not fully understood. Here we studied the potential role of TUG, a widely expressed protein containing a UBX domain, to control mammalian p97. In HEK293 cells, the vast majority of TUG was bound to p97. Surprisingly, the TUG UBX domain was neither necessary nor sufficient for this interaction. Rather, an extended sequence, comprising three regions of TUG, bound to the p97 N-terminal domain. The TUG C terminus resembled the Arabidopsis protein PUX1. Similar to the previously described action of PUX1 on AtCDC48, TUG caused the conversion of p97 hexamers into monomers. Hexamer disassembly was stoichiometric rather than catalytic and was not greatly affected by the p97 ATP-binding state or by TUG N-terminal regions in vitro. In HeLa cells, TUG localized to the endoplasmic reticulum-to-Golgi intermediate compartment and endoplasmic reticulum exit sites. Although siRNA-mediated TUG depletion had no marked effect on total ubiquitylated proteins or p97 localization, TUG overexpression caused an accumulation of ubiquitylated substrates and targeted both TUG and p97 to the nucleus. A physiologic role of TUG was revealed by siRNA-mediated depletion, which showed that TUG is required for efficient reassembly of the Golgi complex after brefeldin A removal. Together, these data support a model in which TUG controls p97 oligomeric status at a particular location in the early secretory pathway and in which this process regulates membrane trafficking in various cell types.
Figures



References
-
- White S. R., Lauring B. (2007) AAA+ ATPases. Achieving diversity of function with conserved machinery. Traffic 8, 1657–1667 - PubMed
-
- Brunger A. T., DeLaBarre B. (2003) NSF and p97/VCP. Similar at first, different at last. FEBS Lett. 555, 126–133 - PubMed
-
- Yeung H. O., Kloppsteck P., Niwa H., Isaacson R. L., Matthews S., Zhang X., Freemont P. S. (2008) Insights into adaptor binding to the AAA protein p97. Biochem. Soc. Trans. 36, 62–67 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

