Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome
- PMID: 22207837
- PMCID: PMC3245647
- DOI: 10.3389/fncel.2011.00029
Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome
Abstract
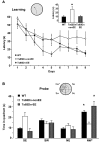
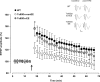
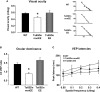
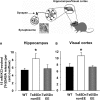
Down syndrome (DS) is the most common genetic disorder associated with mental retardation. It has been repeatedly shown that Ts65Dn mice, the prime animal model for DS, have severe cognitive and neural plasticity defects due to excessive inhibition. We report that increasing sensory-motor stimulation in adulthood through environmental enrichment (EE) reduces brain inhibition levels and promotes recovery of spatial memory abilities, hippocampal synaptic plasticity, and visual functions in adult Ts65Dn mice.
Keywords: Down syndrome; GABAergic inhibition; Ts65Dn mice; cerebral plasticity; environmental enrichment.
Figures
Similar articles
-
Fluoxetine in adulthood normalizes GABA release and rescues hippocampal synaptic plasticity and spatial memory in a mouse model of Down syndrome.Neurobiol Dis. 2014 Mar;63:12-9. doi: 10.1016/j.nbd.2013.11.010. Epub 2013 Nov 19. Neurobiol Dis. 2014. PMID: 24269730
-
Early environmental therapy rescues brain development in a mouse model of Down syndrome.Neurobiol Dis. 2015 Oct;82:409-419. doi: 10.1016/j.nbd.2015.07.014. Epub 2015 Aug 2. Neurobiol Dis. 2015. PMID: 26244989
-
Neuroanatomical alterations and synaptic plasticity impairment in the perirhinal cortex of the Ts65Dn mouse model of Down syndrome.Neurobiol Dis. 2017 Oct;106:89-100. doi: 10.1016/j.nbd.2017.06.017. Epub 2017 Jun 23. Neurobiol Dis. 2017. PMID: 28651891
-
On the cause of mental retardation in Down syndrome: extrapolation from full and segmental trisomy 16 mouse models.Brain Res Brain Res Rev. 2001 Apr;35(2):115-45. doi: 10.1016/s0926-6410(00)00074-4. Brain Res Brain Res Rev. 2001. PMID: 11336779 Review.
-
The GABAergic Hypothesis for Cognitive Disabilities in Down Syndrome.Front Cell Neurosci. 2017 Mar 7;11:54. doi: 10.3389/fncel.2017.00054. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28326014 Free PMC article. Review.
Cited by
-
Fluoxetine increases plasticity and modulates the proteomic profile in the adult mouse visual cortex.Sci Rep. 2015 Jul 24;5:12517. doi: 10.1038/srep12517. Sci Rep. 2015. PMID: 26205348 Free PMC article.
-
Cilostazol, a Phosphodiesterase 3 Inhibitor, Moderately Attenuates Behaviors Depending on Sex in the Ts65Dn Mouse Model of Down Syndrome.Front Aging Neurosci. 2020 Apr 21;12:106. doi: 10.3389/fnagi.2020.00106. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32372946 Free PMC article.
-
Disruption of Critical Period Plasticity in a Mouse Model of Neurofibromatosis Type 1.J Neurosci. 2020 Jul 8;40(28):5495-5509. doi: 10.1523/JNEUROSCI.2235-19.2020. Epub 2020 Jun 11. J Neurosci. 2020. PMID: 32527982 Free PMC article.
-
Principal Component Analysis of the Effects of Environmental Enrichment and (-)-epigallocatechin-3-gallate on Age-Associated Learning Deficits in a Mouse Model of Down Syndrome.Front Behav Neurosci. 2015 Dec 11;9:330. doi: 10.3389/fnbeh.2015.00330. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26696850 Free PMC article.
-
Prospects for improving brain function in individuals with Down syndrome.CNS Drugs. 2013 Sep;27(9):679-702. doi: 10.1007/s40263-013-0089-3. CNS Drugs. 2013. PMID: 23821040 Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

