The clinical assessment and remote administration tablet
- PMID: 22207845
- PMCID: PMC3246293
- DOI: 10.3389/fninf.2011.00031
The clinical assessment and remote administration tablet
Abstract
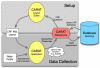
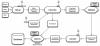
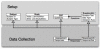
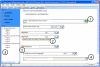
Electronic data capture of case report forms, demographic, neuropsychiatric, or clinical assessments, can vary from scanning hand-written forms into databases to fully electronic systems. Web-based forms can be extremely useful for self-assessment; however, in the case of neuropsychiatric assessments, self-assessment is often not an option. The clinician often must be the person either summarizing or making their best judgment about the subject's response in order to complete an assessment, and having the clinician turn away to type into a web browser may be disruptive to the flow of the interview. The Mind Research Network has developed a prototype for a software tool for the real-time acquisition and validation of clinical assessments in remote environments. We have developed the clinical assessment and remote administration tablet on a Microsoft Windows PC tablet system, which has been adapted to interact with various data models already in use in several large-scale databases of neuroimaging studies in clinical populations. The tablet has been used successfully to collect and administer clinical assessments in several large-scale studies, so that the correct clinical measures are integrated with the correct imaging and other data. It has proven to be incredibly valuable in confirming that data collection across multiple research groups is performed similarly, quickly, and with accountability for incomplete datasets. We present the overall architecture and an evaluation of its use.
Keywords: case report forms; clinical assessments; electronic data capture.
Figures


Similar articles
-
Pivot/Remote: a distributed database for remote data entry in multi-center clinical trials.Medinfo. 1995;8 Pt 2:1097. Medinfo. 1995. PMID: 8591379
-
Optimizing Electronic Capture of Clinical Outcome Assessment Data in Clinical Trials: The Case of Patient-Reported Endpoints.Ther Innov Regul Sci. 2015 Nov;49(6):797-804. doi: 10.1177/2168479015609102. Ther Innov Regul Sci. 2015. PMID: 30222384
-
Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping.J Vis Exp. 2012 Jun 26;(64):3993. doi: 10.3791/3993. J Vis Exp. 2012. PMID: 22782131 Free PMC article.
-
Development of a Smart Mobile Data Module for Fetal Monitoring in E-Healthcare.J Med Syst. 2018 Mar 23;42(5):83. doi: 10.1007/s10916-018-0938-1. J Med Syst. 2018. PMID: 29572752
-
The effectiveness of internet-based e-learning on clinician behavior and patient outcomes: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Jan;13(1):52-64. doi: 10.11124/jbisrir-2015-1919. JBI Database System Rev Implement Rep. 2015. PMID: 26447007
Cited by
-
Computational neuroscience and neuroinformatics: Recent progress and resources.J Biosci. 2018 Dec;43(5):1037-1054. J Biosci. 2018. PMID: 30541962 Review.
-
The Function Biomedical Informatics Research Network Data Repository.Neuroimage. 2016 Jan 1;124(Pt B):1074-1079. doi: 10.1016/j.neuroimage.2015.09.003. Epub 2015 Sep 11. Neuroimage. 2016. PMID: 26364863 Free PMC article.
-
Automated collection of imaging and phenotypic data to centralized and distributed data repositories.Front Neuroinform. 2014 Jun 5;8:60. doi: 10.3389/fninf.2014.00060. eCollection 2014. Front Neuroinform. 2014. PMID: 24926252 Free PMC article.
-
Project, toolkit, and database of neuroinformatics ecosystem: A summary of previous studies on "Frontiers in Neuroinformatics".Front Neuroinform. 2022 Sep 26;16:902452. doi: 10.3389/fninf.2022.902452. eCollection 2022. Front Neuroinform. 2022. PMID: 36225654 Free PMC article. Review.
-
Software and hardware infrastructure for research in electrophysiology.Front Neuroinform. 2014 Mar 7;8:20. doi: 10.3389/fninf.2014.00020. eCollection 2014. Front Neuroinform. 2014. PMID: 24639646 Free PMC article.
References
-
- Bockholt H. J., Scully M., Courtney W., Rachakonda S., Scott A., Caprihan A., Fries J., Kalyanam R., Segall J. M., de la Garza R., Lane S., Calhoun V. D. (2010). Mining the mind research network: a novel framework for exploring large scale, heterogeneous translational neuroscience research data sources. Front. Neuroinform. 3:36.10.3389/neuro.11.036.2009 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

