Expression of the Adenovirus Early Gene 1A Transcription-Repression Domain Alone Downregulates HER2 and Results in the Death of Human Breast Cancer Cells Upregulated for the HER2 Proto-Oncogene
- PMID: 22207899
- PMCID: PMC3218411
- DOI: 10.1177/1947601911426570
Expression of the Adenovirus Early Gene 1A Transcription-Repression Domain Alone Downregulates HER2 and Results in the Death of Human Breast Cancer Cells Upregulated for the HER2 Proto-Oncogene
Abstract
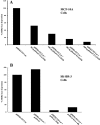
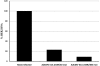
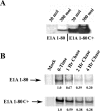
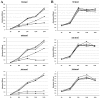
Adenovirus (Ad) early gene 1A 243 residue protein (E1A 243R) possesses a potent transcription-repression function within the N-terminal 80 amino acids (E1A 1-80). We examined the ability of E1A 243R and E1A 1-80 to repress transcription of both an exogenous and the endogenous HER2 promoter in a human breast cancer cell line upregulated for the HER2 proto-oncogene (SK-BR-3). Both moieties repressed HER2 expression by over 90%. When E1A 1-80 was expressed from a nonreplicative Ad vector, levels of expression were lower than anticipated. Addition of nonspecific sequences to the E1A 1-80 C-terminus (E1A 1-80 C+) enhanced its expression 10- to 20-fold. Because "oncogene addiction" suggests that repression of HER2 could kill HER2 upregulated cells, we examined the ability of full-length E1A 243R and E1A 1-80 C+ delivered by an Ad vector to kill HER2 upregulated SK-BR-3 cells. Expression of both E1A 243R and E1A 1-80 C+ killed SK-BR-3 cells but not normal breast cells. E1A 1-80 C+ is a particularly effective killer of SK-BR-3 cells. At 144 h post infection, over 85% of SK-BR-3 cells were killed by a 100 moi of the Ad vector expressing E1A 1-80 C+. As controls, Ad vectors expressing E1A 243R with deletion of all known functional domains or expressing unrelated β-galactosidase had no effect. Three additional human breast cancer cells lines reported to be upregulated for HER2 or another EGF family member (EGFR) were found to be efficiently killed by expression of E1A 1-80 C+, whereas three additional "normal" cell lines (two derived from breast and one from foreskin) were not. The ability of the E1A transcription-repression domain alone to kill HER2 upregulated breast cancer cells has potential for development of therapies for treatment of aggressive human breast cancers and potentially other human cancers that overexpress HER2.
Keywords: E1A; HER2; adenovirus; oncogene addiction; transcription repression.
Conflict of interest statement
The authors declared no conflicts of interest with respect to the authorship and/or publication of the article.
Figures
Similar articles
-
Ad E1A 243R oncoprotein promotes association of proto-oncogene product MYC with the NuA4/Tip60 complex via the E1A N-terminal repression domain.Virology. 2016 Dec;499:178-184. doi: 10.1016/j.virol.2016.09.005. Epub 2016 Sep 22. Virology. 2016. PMID: 27664947 Free PMC article.
-
The adenoviral E1A N-terminal domain represses MYC transcription in human cancer cells by targeting both p300 and TRRAP and inhibiting MYC promoter acetylation of H3K18 and H4K16.Genes Cancer. 2016 Mar;7(3-4):98-109. doi: 10.18632/genesandcancer.99. Genes Cancer. 2016. PMID: 27382434 Free PMC article.
-
TATA-dependent repression of human immunodeficiency virus type-1 transcription by the adenovirus E1A 243R oncoprotein.Oncogene. 1996 Feb 15;12(4):819-26. Oncogene. 1996. PMID: 8632904
-
Dual action of the adenovirus E1A 243R oncoprotein on the human proliferating cell nuclear antigen promoter: repression of transcriptional activation by p53.Oncogene. 1999 Dec 16;18(54):7825-33. doi: 10.1038/sj.onc.1203294. Oncogene. 1999. PMID: 10618724
-
Targeting HER2: recent developments and future directions for breast cancer patients.Semin Oncol. 2001 Dec;28(6 Suppl 18):21-9. doi: 10.1053/sonc.2001.29724. Semin Oncol. 2001. PMID: 11774202 Review.
Cited by
-
Personalizing therapies for gastric cancer: molecular mechanisms and novel targeted therapies.World J Gastroenterol. 2013 Oct 14;19(38):6383-97. doi: 10.3748/wjg.v19.i38.6383. World J Gastroenterol. 2013. PMID: 24151357 Free PMC article. Review.
-
Prognostic Significance of Prostaglandin-Endoperoxide Synthase-2 Expressions in Human Breast Carcinoma: A Multiomic Approach.Cancer Inform. 2020 Nov 6;19:1176935120969696. doi: 10.1177/1176935120969696. eCollection 2020. Cancer Inform. 2020. PMID: 33223820 Free PMC article.
-
The adenovirus E1A N-terminal repression domain represses transcription from a chromatin template in vitro.Virology. 2012 Jun 20;428(1):70-5. doi: 10.1016/j.virol.2012.03.021. Epub 2012 Apr 21. Virology. 2012. PMID: 22521914 Free PMC article.
-
Ad E1A 243R oncoprotein promotes association of proto-oncogene product MYC with the NuA4/Tip60 complex via the E1A N-terminal repression domain.Virology. 2016 Dec;499:178-184. doi: 10.1016/j.virol.2016.09.005. Epub 2016 Sep 22. Virology. 2016. PMID: 27664947 Free PMC article.
-
The adenoviral E1A N-terminal domain represses MYC transcription in human cancer cells by targeting both p300 and TRRAP and inhibiting MYC promoter acetylation of H3K18 and H4K16.Genes Cancer. 2016 Mar;7(3-4):98-109. doi: 10.18632/genesandcancer.99. Genes Cancer. 2016. PMID: 27382434 Free PMC article.
References
-
- Choudhury A, Charo J, Parapuram SK, et al. Small interfering RNA (siRNA) inhibits the expression of the Her2/Neu gene, upregulates HLA class I and induces apoptosis of Her2Neu positive tumor cell lines. Int J Cancer. 2003;108:71-7 - PubMed
-
- Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63-4 - PubMed
-
- Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077-80 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
