Overcoming burdens in the regulation of clinical research in children. Proceedings of a consensus conference, in historical context
- PMID: 22208165
- PMCID: PMC3276425
- DOI: 10.1186/1687-9856-2011-19
Overcoming burdens in the regulation of clinical research in children. Proceedings of a consensus conference, in historical context
Abstract
Background: Many investigators are concerned that the modes of implementation and enforcement of the federal regulations designed to protect children are unduly impeding pediatric clinical research.
Objective: To assess regulatory impediments to clinical research involving children and to develop recommendations to ameliorate them.
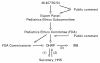
Participants: The Pediatric Endocrine Society and The Endocrine Society convened a consensus conference involving experts and stakeholders in patient-oriented research involving children and adolescents in 2008.
Consensus process: Following presentations that reviewed problematic issues around key regulations, participants divided into working groups to develop potential solutions that could be adopted at local and federal levels. Presentations to the full assembly were then debated. A writing committee then drafted a summary of the discussions and main conclusions, placing them in historical context, and submitted it to all participants for comment with the aim of developing consensus.
Conclusions: Recommendations designed to facilitate the ethical conduct of research involving children addressed the interpretation of ambiguous regulatory terms such as "minimal risk" and "condition" and called for the development by professional societies of best practice primers for common research procedures that would be informative to both investigators and institutional review boards. A call was issued for improved guidance from the Office for Human Research Protections and Food and Drug Administration as well as for the development by professional societies of a process to monitor progress in improving human subject research regulation. Finally, a need for systematic research to define the nature and extent of institutional obstacles to pediatric research was recognized.
Figures
Similar articles
-
American Society of Clinical Oncology policy statement: oversight of clinical research.J Clin Oncol. 2003 Jun 15;21(12):2377-86. doi: 10.1200/JCO.2003.04.026. Epub 2003 Apr 29. J Clin Oncol. 2003. PMID: 12721281
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The International Liaison Committee on Resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients: pediatric basic and advanced life support.Pediatrics. 2006 May;117(5):e955-77. doi: 10.1542/peds.2006-0206. Epub 2006 Apr 17. Pediatrics. 2006. PMID: 16618790
-
Informed consent in emergency research. Consensus statement from the Coalition Conference of Acute Resuscitation and Critical Care Researchers.JAMA. 1995 Apr 26;273(16):1283-7. doi: 10.1001/jama.273.16.1283. JAMA. 1995. PMID: 7715041 Review.
-
Developing Recommendations for Oversight of Patient-Centered Outcomes Research—The PCOROS Study [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Aug. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Aug. PMID: 38556971 Free Books & Documents. Review.
Cited by
-
Evidence for Increased 5α-Reductase Activity During Early Childhood in Daughters of Women With Polycystic Ovary Syndrome.J Clin Endocrinol Metab. 2016 May;101(5):2069-75. doi: 10.1210/jc.2015-3926. Epub 2016 Mar 18. J Clin Endocrinol Metab. 2016. PMID: 26990942 Free PMC article.
-
Clinical review: Adolescent anovulation: maturational mechanisms and implications.J Clin Endocrinol Metab. 2013 Sep;98(9):3572-83. doi: 10.1210/jc.2013-1770. Epub 2013 Aug 2. J Clin Endocrinol Metab. 2013. PMID: 23913942 Free PMC article. Review.
-
Persistent apparent pancreatic β-cell defects in premenarchal PCOS relatives.J Clin Endocrinol Metab. 2014 Oct;99(10):3855-62. doi: 10.1210/jc.2014-1474. Epub 2014 Jul 16. J Clin Endocrinol Metab. 2014. PMID: 25029420 Free PMC article.
-
Commentary: Launch of a quality improvement network for evidence-based management of uncommon pediatric endocrine disorders: Turner syndrome as a prototype.J Clin Endocrinol Metab. 2015 Apr;100(4):1234-6. doi: 10.1210/jc.2014-3845. J Clin Endocrinol Metab. 2015. PMID: 25844763 Free PMC article.
References
-
- Sung NS, Crowley WF Jr, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Korn A, Rimoin D. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. - DOI - PubMed
-
- Office for Human Research Protections (OHRP) Special protections for children as research subjects, U.S. Department of Health and Human Services. 2008. http://www.hhs.gov/ohrp/policy/populations/children.html
LinkOut - more resources
Full Text Sources
Miscellaneous

