Cys-loop receptor channel blockers also block GLIC
- PMID: 22208189
- PMCID: PMC3244065
- DOI: 10.1016/j.bpj.2011.10.055
Cys-loop receptor channel blockers also block GLIC
Abstract
The Gloeobacter ligand-gated ion channel (GLIC) is a bacterial homolog of vertebrate Cys-loop ligand-gated ion channels. Its pore-lining region in particular has a high sequence homology to these related proteins. Here we use electrophysiology to examine a range of compounds that block the channels of Cys-loop receptors to probe their pharmacological similarity with GLIC. The data reveal that a number of these compounds also block GLIC, although the pharmacological profile is distinct from these other proteins. The most potent compound was lindane, a GABA(A) receptor antagonist, with an IC₅₀ of 0.2 μM. Docking studies indicated two potential binding sites for this ligand in the pore, at the 9' or between the 0' and 2' residues. Similar experiments with picrotoxinin (IC₅₀ = 2.6 μM) and rimantadine (IC₅₀ = 2.6 μM) reveal interactions with 2'Thr residues in the GLIC pore. These locations are strongly supported by mutagenesis data for picrotoxinin and lindane, which are less potent in a T2'S version of GLIC. Overall, our data show that the inhibitory profile of the GLIC pore has considerable overlap with those of Cys-loop receptors, but the GLIC pore has a unique pharmacology.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

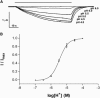
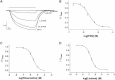


Similar articles
-
Phenylalanine in the pore of the Erwinia ligand-gated ion channel modulates picrotoxinin potency but not receptor function.Biochemistry. 2014 Oct 7;53(39):6183-8. doi: 10.1021/bi5008035. Epub 2014 Sep 19. Biochemistry. 2014. PMID: 25238029 Free PMC article.
-
Structural titration of receptor ion channel GLIC gating by HS-AFM.Proc Natl Acad Sci U S A. 2018 Oct 9;115(41):10333-10338. doi: 10.1073/pnas.1805621115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181288 Free PMC article.
-
Probing residues in the pore-forming (M2) domain of the Cys-loop receptor homologue GLIC reveals some unusual features.Mol Membr Biol. 2015;32(1):26-31. doi: 10.3109/09687688.2015.1023377. Epub 2015 Apr 13. Mol Membr Biol. 2015. PMID: 25865129
-
Structural insights into Cys-loop receptor function and ligand recognition.Biochem Pharmacol. 2013 Oct 15;86(8):1042-53. doi: 10.1016/j.bcp.2013.07.001. Epub 2013 Jul 10. Biochem Pharmacol. 2013. PMID: 23850718 Review.
-
New insights into the structural bases of activation of Cys-loop receptors.J Physiol Paris. 2012 Jan;106(1-2):23-33. doi: 10.1016/j.jphysparis.2011.09.012. Epub 2011 Oct 2. J Physiol Paris. 2012. PMID: 21995938 Review.
Cited by
-
Mapping two neurosteroid-modulatory sites in the prototypic pentameric ligand-gated ion channel GLIC.J Biol Chem. 2018 Feb 23;293(8):3013-3027. doi: 10.1074/jbc.RA117.000359. Epub 2018 Jan 4. J Biol Chem. 2018. PMID: 29301936 Free PMC article.
-
Desensitization mechanism in prokaryotic ligand-gated ion channel.J Biol Chem. 2012 May 25;287(22):18467-77. doi: 10.1074/jbc.M112.348045. Epub 2012 Apr 3. J Biol Chem. 2012. PMID: 22474322 Free PMC article.
-
Ethanol Modulation is Quantitatively Determined by the Transmembrane Domain of Human α1 Glycine Receptors.Alcohol Clin Exp Res. 2015 Jun;39(6):962-8. doi: 10.1111/acer.12735. Epub 2015 May 14. Alcohol Clin Exp Res. 2015. PMID: 25973519 Free PMC article.
-
Perturbation of Critical Prolines in Gloeobacter violaceus Ligand-gated Ion Channel (GLIC) Supports Conserved Gating Motions among Cys-loop Receptors.J Biol Chem. 2016 Mar 18;291(12):6272-80. doi: 10.1074/jbc.M115.694372. Epub 2015 Dec 14. J Biol Chem. 2016. PMID: 26668320 Free PMC article.
-
Macroscopic kinetics of pentameric ligand gated ion channels: comparisons between two prokaryotic channels and one eukaryotic channel.PLoS One. 2013 Nov 19;8(11):e80322. doi: 10.1371/journal.pone.0080322. eCollection 2013. PLoS One. 2013. PMID: 24260369 Free PMC article.
References
-
- Bocquet N., Prado de Carvalho L., Corringer P.J. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. - PubMed
-
- Hilf R.J., Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. - PubMed
-
- Bocquet N., Nury H., Corringer P.J. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

