Aqueous viscosity is the primary source of friction in lipidic pore dynamics
- PMID: 22208191
- PMCID: PMC3244058
- DOI: 10.1016/j.bpj.2011.11.009
Aqueous viscosity is the primary source of friction in lipidic pore dynamics
Abstract
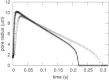
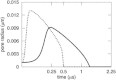
A new theory, to our knowledge, is developed that describes the dynamics of a lipidic pore in a liposome. The equations of the theory capture the experimentally observed three-stage functional form of pore radius over time--stage 1, rapid pore enlargement; stage 2, slow pore shrinkage; and stage 3, rapid pore closure. They also show that lipid flow is kinetically limited by the values of both membrane and aqueous viscosity; therefore, pore evolution is affected by both viscosities. The theory predicts that for a giant liposome, tens of microns in radius, water viscosity dominates over the effects of membrane viscosity. The edge tension of a lipidic pore is calculated by using the theory to quantitatively account for pore kinetics in stage 3, rapid pore closing. This value of edge tension agrees with the value as standardly calculated from the stage of slow pore closure, stage 2. For small, submicron liposomes, membrane viscosity affects pore kinetics, but only if the viscosity of the aqueous solution is comparable to that of distilled water. A first-principle fluid-mechanics calculation of the friction due to aqueous viscosity is in excellent agreement with the friction obtained by applying the new theory to data of previously published experimental results.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Koslov M.M., Markin V.S. A theory of osmotic lysis of lipid vesicles. J. Theor. Biol. 1984;109:17–39. - PubMed
-
- Weaver J.C. Electroporation theory. Concepts and mechanisms. Methods Mol. Biol. 1995;55:3–28. - PubMed
-
- Zhelev D.V., Needham D. Tension-stabilized pores in giant vesicles: determination of pore size and pore line tension. Biochim. Biophys. Acta. 1993;1147:89–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

