Delayed onset of paresis in rats with experimental intramedullary spinal cord gliosarcoma following intratumoral administration of the paclitaxel delivery system OncoGel
- PMID: 22208429
- PMCID: PMC3978141
- DOI: 10.3171/2011.9.SPINE11435
Delayed onset of paresis in rats with experimental intramedullary spinal cord gliosarcoma following intratumoral administration of the paclitaxel delivery system OncoGel
Abstract
Object: Treatment options for anaplastic or malignant intramedullary spinal cord tumors (IMSCTs) remain limited. Paclitaxel has potent cytotoxicity against experimental intracranial gliomas and could be beneficial in the treatment of IMSCTs, but poor CNS penetration and significant toxicity limit its use. Such limitations could be overcome with local intratumoral delivery. Paclitaxel has been previously incorporated into a biodegradable gel depot delivery system (OncoGel) and in this study the authors evaluated the safety of intramedullary injections of OncoGel in rats and its efficacy against an intramedullary rat gliosarcoma.
Methods: Safety of intramedullary OncoGel was tested in 12 Fischer-344 rats using OncoGel concentrations of 1.5 and 6.0 mg/ml (5 μl); median survival and functional motor scores (Basso-Beattie-Bresnahan [BBB] scale) were compared with those obtained with placebo (ReGel) and medium-only injections. Efficacy of OncoGel was tested in 61 Fischer-344 rats implanted with an intramedullary injection of 9L gliosarcoma containing 100,000 cells in 5 μl of medium, and randomized to receive OncoGel administered on the same day (in 32 rats) or 5 days after tumor implantation (in 29 rats) using either 1.5 mg/ml or 3.0 mg/ml doses of paclitaxel. Median survival and BBB scores were compared with those of ReGel-treated and tumor-only rats. Animals were killed after the onset of deficits for histopathological analysis.
Results: OncoGel was safe for intramedullary injection in rats in doses up to 5 μl of 3.0 mg/ml of paclitaxel; a dose of 5 μl of 6.0 mg/ml caused rapid deterioration in BBB scores. OncoGel at concentrations of 1.5 mg/ml and 3.0 mg/ml paclitaxel given on both Day 0 and Day 5 prolonged median survival and preserved BBB scores compared with controls. OncoGel 1.5 mg/ml produced 62.5% long-term survivors when delivered on Day 0. A comparison between the 1.5 mg/ml and the 3.0 mg/ml doses showed higher median survival with the 1.5 mg/ml dose on Day 0, and no differences in median survival or BBB scores after treatment on Day 5.
Conclusions: OncoGel is safe for intramedullary injection in rats in doses up to 5 μl of 3.0 mg/ml, prolongs median survival, and increases functional motor scores in rats challenged with an intramedullary gliosarcoma at the doses tested. This study suggests that locally delivered chemotherapeutic agents could be of temporary benefit in the treatment of malignant IMSCTs under experimental settings.
Figures



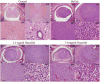
References
-
- Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887–2893. - PubMed
-
- Bagley CA, Bookland MJ, Pindrik JA, Ozmen T, Gokaslan ZL, Witham TF. Local delivery of oncogel delays paresis in rat metastatic spinal tumor model. J Neurosurg Spine. 2007;7:194–198. - PubMed
-
- Balmaceda C. Chemotherapy for intramedullary spinal cord tumors. J Neurooncol. 2000;47:293–307. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

