Comprehensive cardiovascular magnetic resonance of myocardial mechanics in mice using three-dimensional cine DENSE
- PMID: 22208954
- PMCID: PMC3278394
- DOI: 10.1186/1532-429X-13-83
Comprehensive cardiovascular magnetic resonance of myocardial mechanics in mice using three-dimensional cine DENSE
Abstract
Background: Quantitative noninvasive imaging of myocardial mechanics in mice enables studies of the roles of individual genes in cardiac function. We sought to develop comprehensive three-dimensional methods for imaging myocardial mechanics in mice.
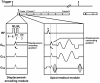
Methods: A 3D cine DENSE pulse sequence was implemented on a 7T small-bore scanner. The sequence used three-point phase cycling for artifact suppression and a stack-of-spirals k-space trajectory for efficient data acquisition. A semi-automatic 2D method was adapted for 3D image segmentation, and automated 3D methods to calculate strain, twist, and torsion were employed. A scan protocol that covered the majority of the left ventricle in a scan time of less than 25 minutes was developed, and seven healthy C57Bl/6 mice were studied.
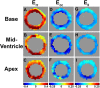
Results: Using these methods, multiphase normal and shear strains were measured, as were myocardial twist and torsion. Peak end-systolic values for the normal strains at the mid-ventricular level were 0.29 ± 0.17, -0.13 ± 0.03, and -0.18 ± 0.14 for E(rr), E(cc), and E(ll), respectively. Peak end-systolic values for the shear strains were 0.00 ± 0.08, 0.04 ± 0.12, and 0.03 ± 0.07 for E(rc), E(rl), and E(cl), respectively. The peak end-systolic normalized torsion was 5.6 ± 0.9°.
Conclusions: Using a 3D cine DENSE sequence tailored for cardiac imaging in mice at 7 T, a comprehensive assessment of 3D myocardial mechanics can be achieved with a scan time of less than 25 minutes and an image analysis time of approximately 1 hour.
Figures




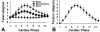
Similar articles
-
Mapping right ventricular myocardial mechanics using 3D cine DENSE cardiovascular magnetic resonance.J Cardiovasc Magn Reson. 2012 Jan 11;14(1):4. doi: 10.1186/1532-429X-14-4. J Cardiovasc Magn Reson. 2012. PMID: 22236389 Free PMC article.
-
Reproducibility of cine displacement encoding with stimulated echoes (DENSE) cardiovascular magnetic resonance for measuring left ventricular strains, torsion, and synchrony in mice.J Cardiovasc Magn Reson. 2013 Aug 27;15(1):71. doi: 10.1186/1532-429X-15-71. J Cardiovasc Magn Reson. 2013. PMID: 23981339 Free PMC article.
-
Imaging three-dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI.Magn Reson Med. 2010 Oct;64(4):1089-97. doi: 10.1002/mrm.22503. Magn Reson Med. 2010. PMID: 20574967 Free PMC article.
-
Quantification of left ventricular volumes, mass, and ejection fraction using cine displacement encoding with stimulated echoes (DENSE) MRI.J Magn Reson Imaging. 2014 Aug;40(2):398-406. doi: 10.1002/jmri.24350. Epub 2013 Oct 29. J Magn Reson Imaging. 2014. PMID: 24923710 Free PMC article.
-
Evaluation of left ventricular torsion by cardiovascular magnetic resonance.J Cardiovasc Magn Reson. 2012 Jul 24;14(1):49. doi: 10.1186/1532-429X-14-49. J Cardiovasc Magn Reson. 2012. PMID: 22827856 Free PMC article. Review.
Cited by
-
Measuring Cardiac Dyssynchrony with DENSE (Displacement Encoding with Stimulated Echoes)-A Systematic Review.Rev Cardiovasc Med. 2023 Sep 18;24(9):261. doi: 10.31083/j.rcm2409261. eCollection 2023 Sep. Rev Cardiovasc Med. 2023. PMID: 39076380 Free PMC article.
-
Validation of in vivo 2D displacements from spiral cine DENSE at 3T.J Cardiovasc Magn Reson. 2015 Jan 30;17(1):5. doi: 10.1186/s12968-015-0119-z. J Cardiovasc Magn Reson. 2015. PMID: 25634468 Free PMC article.
-
Validation of a deep-learning semantic segmentation approach to fully automate MRI-based left-ventricular deformation analysis in cardiotoxicity.Br J Radiol. 2021 Apr 1;94(1120):20201101. doi: 10.1259/bjr.20201101. Epub 2021 Feb 24. Br J Radiol. 2021. PMID: 33571002 Free PMC article.
-
Evaluation of a Novel Finite Element Model of Active Contraction in the Heart.Front Physiol. 2018 Apr 23;9:425. doi: 10.3389/fphys.2018.00425. eCollection 2018. Front Physiol. 2018. PMID: 29740338 Free PMC article.
-
Comprehensive enhanced methodology of an MRI-based automated left-ventricular chamber quantification algorithm and validation in chemotherapy-related cardiotoxicity.J Med Imaging (Bellingham). 2020 Nov;7(6):064002. doi: 10.1117/1.JMI.7.6.064002. Epub 2020 Nov 16. J Med Imaging (Bellingham). 2020. PMID: 33241073 Free PMC article.
References
-
- Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13(3):227–239. - PubMed
-
- Vandsburger MH, French BA, Helm PA, Roy RJ, Kramer CM, Young AA, Epstein FH. Multi-parameter in vivo cardiac magnetic resonance imaging demonstrates normal perfusion reserve despite severely attenuated beta-adrenergic functional response in neuronal nitric oxide synthase knockout mice. Eur Heart J. 2007;28:2792–2798. doi: 10.1093/eurheartj/ehm241. - DOI - PubMed
-
- Gilson WD, Epstein FH, Yang Z, Xu Y, Prasad KM, Toufektsian MC, Laubach VE, French BA. Borderzone contractile dysfunction is transiently attenuated and left ventricular structural remodeling is markedly reduced following reperfused myocardial infarction in inducible nitric oxide synthase knockout mice. J Am Coll Cardiol. 2007;50:1799–1807. doi: 10.1016/j.jacc.2007.07.047. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

