Autoantigenesis: the evolution of protein modifications in autoimmune disease
- PMID: 22209691
- PMCID: PMC3288476
- DOI: 10.1016/j.coi.2011.12.003
Autoantigenesis: the evolution of protein modifications in autoimmune disease
Abstract
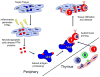
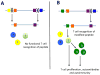
Protein targets in autoimmune disease vary in location, originating within cells as in system lupus erythematosus (SLE), or found on cell surfaces or in extracellular spaces. The term 'autoantigenesis' is first defined here as the changes that arise in self-proteins as they break self-tolerance and trigger autoimmune B and/or T cell responses. As illustrated in many studies, between 50 and 90% of the proteins in the human body acquire post-translational modification. In some cases, it may be that these modifications are necessary for the biological functions of proteins of the cells in which they reside or as extracellular mediators. Summarized herein, it is clear that some post-translational modifications can create new self-antigens by altering immunologic processing and presentation. While many protein modifications exist, we will focus on those created, amplified, or altered in the context of inflammation or other immune system responses. Finally, we will address how post-translational modifications in self-antigens may affect the analyses of B and T cell specificity, current diagnostic techniques, and/or the development of immunotherapies for autoimmune diseases.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Uy R, Wold F. Posttranslational covalent modifications of proteins. Science. 1977;198:890–896. - PubMed
-
- Wold F. In vivo chemical modification of proteins (post-translational modification) Annu Rev Biochem. 1981;50:783–814. - PubMed
-
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nature Immunol. 2001;2:1032–1039. - PubMed
-
- Iversen OJ, Lysvand H, Hagen L. The autoantigen Pso p27: a post-translational modification of SCCA molecules. Autoimmunity. 2011;44:229–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

