NOTCH2 mutations in Alagille syndrome
- PMID: 22209762
- PMCID: PMC3682659
- DOI: 10.1136/jmedgenet-2011-100544
NOTCH2 mutations in Alagille syndrome
Abstract
Background: Alagille syndrome (ALGS) is a dominant, multisystem disorder caused by mutations in the Jagged1 (JAG1) ligand in 94% of patients, and in the NOTCH2 receptor in <1%. There are only two NOTCH2 families reported to date. This study hypothesised that additional NOTCH2 mutations would be present in patients with clinical features of ALGS without a JAG1 mutation.
Methods: The study screened a cohort of JAG1-negative individuals with clinical features suggestive or diagnostic of ALGS for NOTCH2 mutations.
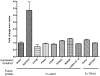
Results: Eight individuals with novel NOTCH2 mutations (six missense, one splicing, and one non-sense mutation) were identified. Three of these patients met classic criteria for ALGS and five patients only had a subset of features. The mutations were distributed across the extracellular (N=5) and intracellular domains (N=3) of the protein. Functional analysis of four missense, one nonsense, and one splicing mutation demonstrated decreased Notch signalling of these proteins. Subjects with NOTCH2 mutations demonstrated highly variable expressivity of the affected systems, as with JAG1 individuals. Liver involvement was universal in NOTCH2 probands and they had a similar prevalence of ophthalmologic and renal anomalies to JAG1 patients. There was a trend towards less cardiac involvement in the NOTCH2 group (60% vs 100% in JAG1). NOTCH2 (+) probands exhibited a significantly decreased penetrance of vertebral abnormalities (10%) and facial features (20%) when compared to the JAG1 (+) cohort.
Conclusions: This work confirms the importance of NOTCH2 as a second disease gene in ALGS and expands the repertoire of the NOTCH2 related disease phenotype.
Figures
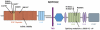

References
-
- Alagille D, Estrada A, Hadchouel M, Gautier M, Odievre M, Dommergues JP. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110:195–200. - PubMed
-
- Berard E, Sarles J, Triolo V, Gagnadoux MF, Wernert F, Hadchouel M, Niaudet P. Renovascular hypertension and vascular anomalies in Alagille syndrome. Pediatr Nephrol. 1998;12:121–4. - PubMed
-
- Harendza S, Hubner CA, Glaser C, Burdelski M, Thaiss F, Hansmann I, Gal A, Stahl RA. Renal failure and hypertension in Alagille syndrome with a novel JAG1 mutation. J Nephrol. 2005;18:312–17. - PubMed
-
- Kamath BM, Spinner NB, Emerick KM, Chudley AE, Booth C, Piccoli DA, Krantz ID. Vascular anomalies in alagille syndrome: a significant cause of morbidity and mortality. Circulation. 2004;109:1354–8. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] - PubMed
-
- Oestreich AE, Sokol RJ, Suchy FJ, Heubi JE. Renal abnormalities in arteriohepatic dysplasia and nonsyndromic intrahepatic biliary hypoplasia. Ann Radiol (Paris) 1983;26:203–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
