The bisretinoids of retinal pigment epithelium
- PMID: 22209824
- PMCID: PMC3288746
- DOI: 10.1016/j.preteyeres.2011.12.001
The bisretinoids of retinal pigment epithelium
Abstract
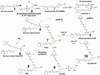
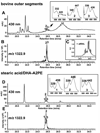
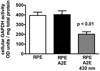
The retina exhibits an inherent autofluorescence that is imaged ophthalmoscopically as fundus autofluorescence. In clinical settings, fundus autofluorescence examination aids in the diagnosis and follow-up of many retinal disorders. Fundus autofluorescence originates from the complex mixture of bisretinoid fluorophores that are amassed by retinal pigment epithelial (RPE) cells as lipofuscin. Unlike the lipofuscin found in other cell-types, this material does not form as a result of oxidative stress. Rather, the formation is attributable to non-enzymatic reactions of vitamin A aldehyde in photoreceptor cells; transfer to RPE occurs upon phagocytosis of photoreceptor outer segments. These fluorescent pigments accumulate even in healthy photoreceptor cells and are generated as a consequence of the light capturing function of the cells. Nevertheless, the formation of this material is accelerated in some retinal disorders including recessive Stargardt disease and ELOVL4-related retinal degeneration. As such, these bisretinoid side-products are implicated in the disease processes that threaten vision. In this article, we review our current understanding of the composition of RPE lipofuscin, the structural characteristics of the various bisretinoids, their related spectroscopic features and the biosynthetic pathways by which they form. We will revisit factors known to influence the extent of the accumulation and therapeutic strategies being used to limit bisretinoid formation. Given their origin from vitamin A aldehyde, an isomer of the visual pigment chromophore, it is not surprising that the bisretinoids of retina are light sensitive molecules. Accordingly, we will discuss recent findings that implicate the photodegradation of bisretinoid in the etiology of age-related macular degeneration.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





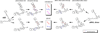
References
-
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. - PubMed
-
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, Kim SR, Maguire AM, Rex TS, Di Vicino U, Cutillo L, Sparrow JR, Williams DS, Bennett J, Auricchio A. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. - PMC - PubMed
-
- Anderson RE, Maude MB. Phospholipids of bovine outer segments. Biochemistry. 1970;9:3624–3628. - PubMed
-
- Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

