Salsolinol stimulates dopamine neurons in slices of posterior ventral tegmental area indirectly by activating μ-opioid receptors
- PMID: 22209890
- PMCID: PMC3310697
- DOI: 10.1124/jpet.111.186833
Salsolinol stimulates dopamine neurons in slices of posterior ventral tegmental area indirectly by activating μ-opioid receptors
Abstract
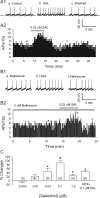
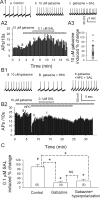
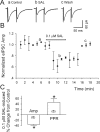
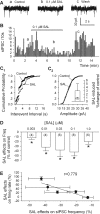
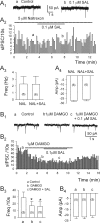
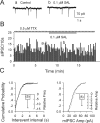
Previous studies in vivo have shown that salsolinol, the condensation product of acetaldehyde and dopamine, has properties that may contribute to alcohol abuse. Although opioid receptors, especially the μ-opioid receptors (MORs), may be involved, the cellular mechanisms mediating the effects of salsolinol have not been fully explored. In the current study, we used whole-cell patch-clamp recordings to examine the effects of salsolinol on dopamine neurons of the ventral tegmental area (VTA) in acute brain slices from Sprague-Dawley rats. Salsolinol (0.01-1 μM) dose-dependently and reversibly increased the ongoing firing of dopamine neurons; this effect was blocked by naltrexone, an antagonist of MORs, and gabazine, an antagonist of GABA(A) receptors. We further showed that salsolinol reduced the frequency without altering the amplitude of spontaneous GABA(A) receptor-mediated inhibitory postsynaptic currents in dopamine neurons. The salsolinol-induced reduction was blocked by both naltrexone and [D-Ala2,N-Me-Phe4,Gly5-ol]enkephalin, an agonist of MORs. Thus, salsolinol excites VTA-dopamine neurons indirectly by activating MORs, which inhibit GABA neurons in the VTA. This form of disinhibition seems to be a novel mechanism underlying the effects of salsolinol.
Figures
Similar articles
-
Salsolinol facilitates glutamatergic transmission to dopamine neurons in the posterior ventral tegmental area of rats.PLoS One. 2012;7(5):e36716. doi: 10.1371/journal.pone.0036716. Epub 2012 May 10. PLoS One. 2012. PMID: 22590592 Free PMC article.
-
Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of mu-opioid receptors.Neuroscience. 2008 Apr 22;153(1):240-8. doi: 10.1016/j.neuroscience.2008.01.040. Epub 2008 Feb 6. Neuroscience. 2008. PMID: 18343590 Free PMC article.
-
The rostromedial tegmental nucleus RMTg is not a critical site for ethanol-induced motor activation in rats.Psychopharmacology (Berl). 2023 Oct;240(10):2071-2080. doi: 10.1007/s00213-023-06425-4. Epub 2023 Jul 20. Psychopharmacology (Berl). 2023. PMID: 37474756 Free PMC article.
-
Locomotor stimulant effects of acute and repeated intrategmental injections of salsolinol in rats: role of mu-opioid receptors.Psychopharmacology (Berl). 2010 Mar;209(1):1-11. doi: 10.1007/s00213-009-1751-9. Psychopharmacology (Berl). 2010. PMID: 20084370
-
Progress in opioid reward research: From a canonical two-neuron hypothesis to two neural circuits.Pharmacol Biochem Behav. 2021 Jan;200:173072. doi: 10.1016/j.pbb.2020.173072. Epub 2020 Nov 20. Pharmacol Biochem Behav. 2021. PMID: 33227308 Free PMC article. Review.
Cited by
-
A Survey on Potentially Beneficial and Hazardous Bioactive Compounds in Cocoa Powder Samples Sourced from the European Market.Foods. 2024 Aug 3;13(15):2457. doi: 10.3390/foods13152457. Foods. 2024. PMID: 39123648 Free PMC article.
-
Involvement of the endogenous opioid system in the psychopharmacological actions of ethanol: the role of acetaldehyde.Front Behav Neurosci. 2013 Jul 31;7:93. doi: 10.3389/fnbeh.2013.00093. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23914161 Free PMC article.
-
Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules.Antioxidants (Basel). 2020 Sep 4;9(9):830. doi: 10.3390/antiox9090830. Antioxidants (Basel). 2020. PMID: 32899889 Free PMC article. Review.
-
Salsolinol modulation of dopamine neurons.Front Behav Neurosci. 2013 May 24;7:52. doi: 10.3389/fnbeh.2013.00052. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23745110 Free PMC article.
-
Behavioral and biochemical evidence of the role of acetaldehyde in the motivational effects of ethanol.Front Behav Neurosci. 2013 Jul 15;7:86. doi: 10.3389/fnbeh.2013.00086. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23874276 Free PMC article.
References
-
- Brodie MS, Shefner SA, Dunwiddie TV. (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508:65–69 - PubMed
-
- Correa M, Salamone JD, Segovia KN, Pardo M, Longoni R, Spina L, Peana AT, Vinci S, Acquas E. (2011) Piecing together the puzzle of acetaldehyde as a neuroactive agent. Neurosci Biobehav Rev 36:404–430 - PubMed
-
- David V, Durkin TP, Cazala P. (1997) Self-administration of the GABAA antagonist bicuculline into the ventral tegmental area in mice: dependence on D2 dopaminergic mechanisms. Psychopharmacology (Berl) 130:85–90 - PubMed
-
- Davis VE, Walsh MJ. (1970) Alcohol, amines, and alkaloids: a possible biochemical basis for alcohol addiction. Science 167:1005–1007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

