Nuclear export of proteins and drug resistance in cancer
- PMID: 22209898
- PMCID: PMC4521586
- DOI: 10.1016/j.bcp.2011.12.016
Nuclear export of proteins and drug resistance in cancer
Abstract
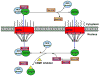
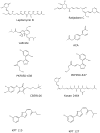
The intracellular location of a protein is crucial to its normal functioning in a cell. Cancer cells utilize the normal processes of nuclear-cytoplasmic transport through the nuclear pore complex of a cell to effectively evade anti-neoplastic mechanisms. CRM1-mediated export is increased in various cancers. Proteins that are exported in cancer include tumor-suppressive proteins such as retinoblastoma, APC, p53, BRAC1, FOXO proteins, INI1/hSNF5, galectin-3, Bok, nucleophosmin, RASSF2, Merlin, p21(CIP), p27(KIP1), N-WASP/FAK, estradiol receptor and Tob, drug targets topoisomerase I and IIα and BCR-ABL, and the molecular chaperone protein Hsp90. Here, we review in detail the current processes and known structures involved in the export of a protein through the nuclear pore complex. We also discuss the export receptor molecule CRM1 and its binding to the leucine-rich nuclear export signal of the cargo protein and the formation of a nuclear export trimer with RanGTP. The therapeutic potential of various CRM1 inhibitors will be addressed, including leptomycin B, ratjadone, KOS-2464, and specific small molecule inhibitors of CRM1, N-azolylacrylate analogs, FOXO export inhibitors, valtrate, acetoxychavicol acetate, CBS9106, and SINE inhibitors. We will also discuss examples of how drug resistance may be reversed by targeting the exported proteins topoisomerase IIα, BCR-ABL, and galectin-3. As effective and less toxic CRM1 export inhibitors become available, they may be used as both single agents and in combination with current chemotherapeutic drugs. We believe that the future development of low-toxicity, small-molecule CRM1 inhibitors may provide a new approach to treating cancer.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–92. - PubMed
-
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. - PubMed
-
- Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloropla-tinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993;268:20116–25. - PubMed
-
- Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

