Frequency selectivity and dopamine-dependence of plasticity at glutamatergic synapses in the subthalamic nucleus
- PMID: 22209920
- PMCID: PMC4245479
- DOI: 10.1016/j.neuroscience.2011.12.027
Frequency selectivity and dopamine-dependence of plasticity at glutamatergic synapses in the subthalamic nucleus
Abstract
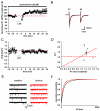
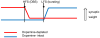
In Parkinson's disease, subthalamic nucleus (STN) neurons burst fire with increased periodicity and synchrony. This may entail abnormal release of glutamate, the major source of which in STN is cortical afferents. Indeed, the cortico-subthalamic pathway is implicated in the emergence of excessive oscillations, which are reduced, as are symptoms, by dopamine-replacement therapy or deep brain stimulation (DBS) targeted to STN. Here we hypothesize that glutamatergic synapses in the STN may be differentially modulated by low-frequency stimulation (LFS) and high-frequency stimulation (HFS), the latter mimicking deep brain stimulation. Recordings of evoked and spontaneous excitatory post synaptic currents (EPSCs) were made from STN neurons in brain slices obtained from dopamine-intact and chronically dopamine-depleted adult rats. HFS had no significant effect on evoked (e) EPSC amplitude in dopamine-intact slices (104.4±8.0%) but depressed eEPSCs in dopamine-depleted slices (67.8±6.2%). Conversely, LFS potentiated eEPSCs in dopamine-intact slices (126.4±8.1%) but not in dopamine-depleted slices (106.7±10.0%). Analyses of paired-pulse ratio, coefficient of variation, and spontaneous EPSCs suggest that the depression and potentiation have a presynaptic locus of expression. These results indicate that the synaptic efficacy in dopamine-intact tissue is enhanced by LFS. Furthermore, the synaptic efficacy in dopamine-depleted tissue is depressed by HFS. Therefore the therapeutic effects of DBS in Parkinson's disease appear mediated, in part, by glutamatergic cortico-subthalamic synaptic depression and implicate dopamine-dependent increases in the weight of glutamate synapses, which would facilitate the transfer of pathological oscillations from the cortex.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation.Synapse. 2003 Dec 15;50(4):314-9. doi: 10.1002/syn.10274. Synapse. 2003. PMID: 14556236
-
Connectivity and Dynamics Underlying Synaptic Control of the Subthalamic Nucleus.J Neurosci. 2019 Mar 27;39(13):2470-2481. doi: 10.1523/JNEUROSCI.1642-18.2019. Epub 2019 Jan 30. J Neurosci. 2019. PMID: 30700533 Free PMC article.
-
D2-like dopamine receptor-mediated modulation of activity-dependent plasticity at GABAergic synapses in the subthalamic nucleus.J Physiol. 2008 Apr 15;586(8):2121-42. doi: 10.1113/jphysiol.2008.151118. Epub 2008 Feb 21. J Physiol. 2008. PMID: 18292127 Free PMC article.
-
Neurotransmitter release from high-frequency stimulation of the subthalamic nucleus.J Neurosurg. 2004 Sep;101(3):511-7. doi: 10.3171/jns.2004.101.3.0511. J Neurosurg. 2004. PMID: 15352610
-
GABAergic control of the subthalamic nucleus.Prog Brain Res. 2007;160:173-88. doi: 10.1016/S0079-6123(06)60010-1. Prog Brain Res. 2007. PMID: 17499114 Review.
Cited by
-
Axonal and synaptic failure suppress the transfer of firing rate oscillations, synchrony and information during high frequency deep brain stimulation.Neurobiol Dis. 2014 Feb;62:86-99. doi: 10.1016/j.nbd.2013.09.006. Epub 2013 Sep 16. Neurobiol Dis. 2014. PMID: 24051279 Free PMC article.
-
High and low frequency stimulation of the subthalamic nucleus induce prolonged changes in subthalamic and globus pallidus neurons.Front Syst Neurosci. 2013 Dec 18;7:73. doi: 10.3389/fnsys.2013.00073. eCollection 2013. Front Syst Neurosci. 2013. PMID: 24391553 Free PMC article.
-
NMDA Receptors Containing the GluN2D Subunit Control Neuronal Function in the Subthalamic Nucleus.J Neurosci. 2015 Dec 2;35(48):15971-83. doi: 10.1523/JNEUROSCI.1702-15.2015. J Neurosci. 2015. PMID: 26631477 Free PMC article.
-
Exploration behavior after reversals is predicted by STN-GPe synaptic plasticity in a basal ganglia model.iScience. 2023 Apr 11;26(5):106599. doi: 10.1016/j.isci.2023.106599. eCollection 2023 May 19. iScience. 2023. PMID: 37250300 Free PMC article.
-
Bursting activity of substantia nigra pars reticulata neurons in mouse parkinsonism in awake and anesthetized states.Neurobiol Dis. 2015 Mar;75:177-85. doi: 10.1016/j.nbd.2014.12.026. Epub 2015 Jan 6. Neurobiol Dis. 2015. PMID: 25576395 Free PMC article.
References
-
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
-
- Benazzouz A, Gross C, Féger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5(4):382–389. - PubMed
-
- Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55(12 Suppl 6):S13–S16. - PubMed
-
- Bergman H, Wichmann T, Karmon B, Delong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

