Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity
- PMID: 22210313
- PMCID: PMC3266396
- DOI: 10.2337/db11-0305
Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity
Abstract
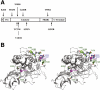
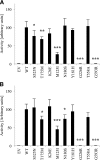
Null mutations in the PCSK1 gene, encoding the proprotein convertase 1/3 (PC1/3), cause recessive monogenic early onset obesity. Frequent coding variants that modestly impair PC1/3 function mildly increase the risk for common obesity. The aim of this study was to determine the contribution of rare functional PCSK1 mutations to obesity. PCSK1 exons were sequenced in 845 nonconsanguineous extremely obese Europeans. Eight novel nonsynonymous PCSK1 mutations were identified, all heterozygous. Seven mutations had a deleterious effect on either the maturation or the enzymatic activity of PC1/3 in cell lines. Of interest, five of these novel mutations, one of the previously described frequent variants (N221D), and the mutation found in an obese mouse model (N222D), affect residues at or near the structural calcium binding site Ca-1. The prevalence of the newly identified mutations was assessed in 6,233 obese and 6,274 lean European adults and children, which showed that carriers of any of these mutations causing partial PCSK1 deficiency had an 8.7-fold higher risk to be obese than wild-type carriers. These results provide the first evidence of an increased risk of obesity in heterozygous carriers of mutations in the PCSK1 gene. Furthermore, mutations causing partial PCSK1 deficiency are present in 0.83% of extreme obesity phenotypes.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

