Cortical plasticity for visuospatial processing and object recognition in deaf and hearing signers
- PMID: 22210355
- PMCID: PMC3288167
- DOI: 10.1016/j.neuroimage.2011.12.031
Cortical plasticity for visuospatial processing and object recognition in deaf and hearing signers
Abstract
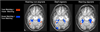
Experience-dependent plasticity in deaf participants has been shown in a variety of studies focused on either the dorsal or ventral aspects of the visual system, but both systems have never been investigated in concert. Using functional magnetic resonance imaging (fMRI), we investigated functional plasticity for spatial processing (a dorsal visual pathway function) and for object processing (a ventral visual pathway function) concurrently, in the context of differing sensory (auditory deprivation) and language (use of a signed language) experience. During scanning, deaf native users of American Sign Language (ASL), hearing native ASL users, and hearing participants without ASL experience attended to either the spatial arrangement of frames containing objects or the identity of the objects themselves. These two tasks revealed the expected dorsal/ventral dichotomy for spatial versus object processing in all groups. In addition, the object identity matching task contained both face and house stimuli, allowing us to examine category-selectivity in the ventral pathway in all three participant groups. When contrasting the groups we found that deaf signers differed from the two hearing groups in dorsal pathway parietal regions involved in spatial cognition, suggesting sensory experience-driven plasticity. Group differences in the object processing system indicated that responses in the face-selective right lateral fusiform gyrus and anterior superior temporal cortex were sensitive to a combination of altered sensory and language experience, whereas responses in the amygdala were more closely tied to sensory experience. By selectively engaging the dorsal and ventral visual pathways within participants in groups with different sensory and language experiences, we have demonstrated that these experiences affect the function of both of these systems, and that certain changes are more closely tied to sensory experience, while others are driven by the combination of sensory and language experience.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no financial conflict of interests.
Figures


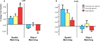

Similar articles
-
Neural Activity During Mental Rotation in Deaf Signers: The Influence of Long-Term Sign Language Experience.Ear Hear. 2018 Sep/Oct;39(5):1015-1024. doi: 10.1097/AUD.0000000000000540. Ear Hear. 2018. PMID: 29298164
-
How Auditory Experience Differentially Influences the Function of Left and Right Superior Temporal Cortices.J Neurosci. 2017 Sep 27;37(39):9564-9573. doi: 10.1523/JNEUROSCI.0846-17.2017. Epub 2017 Aug 18. J Neurosci. 2017. PMID: 28821674 Free PMC article.
-
CNS activation and regional connectivity during pantomime observation: no engagement of the mirror neuron system for deaf signers.Neuroimage. 2010 Jan 1;49(1):994-1005. doi: 10.1016/j.neuroimage.2009.08.001. Epub 2009 Aug 11. Neuroimage. 2010. PMID: 19679192 Free PMC article.
-
Human brain plasticity: evidence from sensory deprivation and altered language experience.Prog Brain Res. 2002;138:177-88. doi: 10.1016/S0079-6123(02)38078-6. Prog Brain Res. 2002. PMID: 12432770 Review.
-
Processing a dynamic visual-spatial language: psycholinguistic studies of American Sign Language.J Psycholinguist Res. 1993 Mar;22(2):153-87. doi: 10.1007/BF01067829. J Psycholinguist Res. 1993. PMID: 8366475 Review.
Cited by
-
Increased Right Posterior STS Recruitment Without Enhanced Directional-Tuning During Tactile Motion Processing in Early Deaf Individuals.Front Neurosci. 2020 Aug 25;14:864. doi: 10.3389/fnins.2020.00864. eCollection 2020. Front Neurosci. 2020. PMID: 32982667 Free PMC article.
-
Simultaneous perception of a spoken and a signed language: The brain basis of ASL-English code-blends.Brain Lang. 2015 Aug;147:96-106. doi: 10.1016/j.bandl.2015.05.006. Epub 2015 Jul 10. Brain Lang. 2015. PMID: 26177161 Free PMC article.
-
Modifications of Visual Field Asymmetries for Face Categorization in Early Deaf Adults: A Study With Chimeric Faces.Front Psychol. 2017 Jan 20;8:30. doi: 10.3389/fpsyg.2017.00030. eCollection 2017. Front Psychol. 2017. PMID: 28163692 Free PMC article.
-
Auditory deprivation affects biases of visuospatial attention as measured by line bisection.Exp Brain Res. 2014 Sep;232(9):2767-73. doi: 10.1007/s00221-014-3960-7. Epub 2014 Apr 26. Exp Brain Res. 2014. PMID: 24770861
-
Altered regional and circuit resting-state activity associated with unilateral hearing loss.PLoS One. 2014 May 1;9(5):e96126. doi: 10.1371/journal.pone.0096126. eCollection 2014. PLoS One. 2014. PMID: 24788317 Free PMC article.
References
-
- Armstrong BA, Neville HJ, Hillyard SA, Mitchell TV. Auditory deprivation affects processing of motion, but not color. Cogn Brain Res. 2002;14(3):422–434. - PubMed
-
- Arnold P, Mills M. Memory for faces, shoes, and objects by deaf and hearing signers and hearing nonsigners. J Psycholinguist Res. 2001;30(2):185–195. - PubMed
-
- Arnold P, Murray C. Memory for faces and objects by deaf and hearing signers and hearing nonsigners. J Psycholinguist Res. 1998;27(4):481–497. - PubMed
-
- Baker CI, Behrmann M, Olson CR. Impact of learning on representation of parts and wholes in monkey inferotemporal cortex. Nat Neurosci. 2002;5(11):1210–1216. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

