A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years
- PMID: 22210571
- PMCID: PMC3263860
- DOI: 10.2337/dc11-1746
A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years
Abstract
Objective: Continuous glucose monitoring (CGM) has been demonstrated to improve glycemic control in adults with type 1 diabetes but less so in children. We designed a study to assess CGM benefit in young children aged 4 to 9 years with type 1 diabetes.
Research design and methods: After a run-in phase, 146 children with type 1 diabetes (mean age 7.5 ± 1.7 years, 64% on pumps, median diabetes duration 3.5 years) were randomly assigned to CGM or to usual care. The primary outcome was reduction in HbA(1c) at 26 weeks by ≥0.5% without the occurrence of severe hypoglycemia.
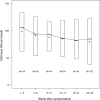
Results: The primary outcome was achieved by 19% in the CGM group and 28% in the control group (P = 0.17). Mean change in HbA(1c) was -0.1% in each group (P = 0.79). Severe hypoglycemia rates were similarly low in both groups. CGM wear decreased over time, with only 41% averaging at least 6 days/week at 26 weeks. There was no correlation between CGM use and change in HbA(1c) (r(s) = -0.09, P = 0.44). CGM wear was well tolerated, and parental satisfaction with CGM was high. However, parental fear of hypoglycemia was not reduced.
Conclusions: CGM in 4- to 9-year-olds did not improve glycemic control despite a high degree of parental satisfaction with CGM. We postulate that this finding may be related in part to limited use of the CGM glucose data in day-to-day management and to an unremitting fear of hypoglycemia. Overcoming the barriers that prevent integration of these critical glucose data into day-to-day management remains a challenge.
Trial registration: ClinicalTrials.gov NCT00760526.
Figures
References
-
- Tamborlane WV, Beck RW, Bode BW, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 - PubMed
-
- Bergenstal RM, Tamborlane WV, Ahmann A, et al. ; STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 - PubMed
-
- Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther 2008;10:377–383 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD041906/HD/NICHD NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- HD-41890-10/HD/NICHD NIH HHS/United States
- HD-41918/HD/NICHD NIH HHS/United States
- HD-41915/HD/NICHD NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- UL1-RR-024139/RR/NCRR NIH HHS/United States
- U10 HD056526/HD/NICHD NIH HHS/United States
- U01 HD041890/HD/NICHD NIH HHS/United States
- U10 HD041908/HD/NICHD NIH HHS/United States
- U10 HD041890/HD/NICHD NIH HHS/United States
- UL1-RR-024979/RR/NCRR NIH HHS/United States
- HD-41906-10/HD/NICHD NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
- HD-56526/HD/NICHD NIH HHS/United States
- U10 HD041918/HD/NICHD NIH HHS/United States
- HD-41908-10/HD/NICHD NIH HHS/United States
- U10 HD041915/HD/NICHD NIH HHS/United States
- UL1-RR-024992/RR/NCRR NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1-RR-025744/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

