Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: the FIELD Study
- PMID: 22210576
- PMCID: PMC3263870
- DOI: 10.2337/dc11-1109
Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: the FIELD Study
Abstract
Objective: Diabetic patients with moderate renal impairment (estimated glomerular filtration rate [eGFR] 30-59 mL/min/1.73 m(2)) are at particular cardiovascular risk. Fenofibrate's safety in these patients is an issue because it may elevate plasma creatinine. Furthermore, guidelines regarding fenofibrate dosing in renal impairment vary internationally. We investigated fenofibrate's effects on cardiovascular and end-stage renal disease (ESRD) events, according to eGFR, in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study.
Research design and methods: Type 2 diabetic patients (aged 50-75 years) with eGFR ≥30 mL/min/1.73 m(2) were randomly allocated to a fixed dose of fenofibrate (200 mg daily) (n = 4,895) or placebo (n = 4,900) for 5 years. Baseline renal function (Modification of Diet in Renal Disease equation) was grouped by eGFR (30-59, 60-89, and ≥90 mL/min/1.73 m(2)). The prespecified outcome was total cardiovascular events (composite of cardiovascular death, myocardial infarction, stroke, and coronary/carotid revascularization). Serious adverse events and instances of ESRD (plasma creatinine >400 μmol/L, dialysis, renal transplant, or renal death) were recorded. Analysis was by intention to treat.
Results: Overall, fenofibrate reduced total cardiovascular events, compared with placebo (hazard ratio 0.89 [95% CI 0.80-0.99]; P = 0.035). This benefit was not statistically different across eGFR groupings (P = 0.2 for interaction) (eGFR 30-59 mL/min/1.73 m(2): 0.68 [0.47-0.97], P = 0.035; eGFR ≥90 mL/min/1.73 m(2): 0.85 [0.70-1.02], P = 0.08). ESRD rates were similar between treatment arms, without adverse safety signals of fenofibrate use in renal impairment.
Conclusions: Patients with type 2 diabetes and moderate renal impairment benefit from long-term fenofibrate, without excess drug-related safety concerns compared with those with no or mild renal impairment. Fenofibrate treatment should not be contraindicated in moderate renal impairment, suggesting that current guidelines may be too restrictive.
Figures
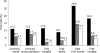
 , 60–89 mL/min/1.73 m2; □, ≥90 mL/min/1.73 m2.
, 60–89 mL/min/1.73 m2; □, ≥90 mL/min/1.73 m2.
References
-
- World Health Organization. Cardiovascular diseases (CVD) [fact sheet online], 2009. Available from http://www.who.int/mediacentre/factsheets/fs317/en/index.html Accessed 3 March 2010
-
- Hemmelgarn BR, Manns BJ, Lloyd A, et al. ; Alberta Kidney Disease Network Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010;303:423–429 - PubMed
-
- Drury PL, Ting R, Zannino D, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 2011;54:32–43 - PubMed
-
- Keech A, Simes RJ, Barter P, et al. ; FIELD Study Investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD Study): randomised controlled trial. Lancet 2005;366:1849–1861 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

