Altered regulation of Escherichia coli biotin biosynthesis in BirA superrepressor mutant strains
- PMID: 22210766
- PMCID: PMC3294782
- DOI: 10.1128/JB.06549-11
Altered regulation of Escherichia coli biotin biosynthesis in BirA superrepressor mutant strains
Abstract
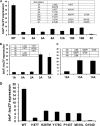
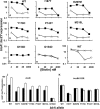
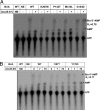
Transcription of the Escherichia coli biotin (bio) operon is directly regulated by the biotin protein ligase BirA, the enzyme that covalently attaches biotin to its cognate acceptor proteins. Binding of BirA to the bio operator requires dimerization of the protein, which is triggered by BirA-catalyzed synthesis of biotinoyl-adenylate (biotinoyl-5'-AMP), the obligatory intermediate of the ligation reaction. Although several aspects of this regulatory system are well understood, no BirA superrepressor mutant strains had been isolated. Such superrepressor BirA proteins would repress the biotin operon transcription in vivo at biotin concentrations well below those needed for repression by wild-type BirA. We isolated mutant strains having this phenotype by a combined selection-screening approach and resolved multiple mutations to give several birA superrepressor alleles, each having a single mutation, all of which showed repression dominant over that of the wild-type allele. All of these mutant strains repressed bio operon transcription in vivo at biotin concentrations that gave derepression of the wild-type strain and retained sufficient ligation activity for growth when overexpressed. All of the strains except that encoding G154D BirA showed derepression of bio operon transcription upon overproduction of a biotin-accepting protein. In BirA, G154D was a lethal mutation in single copy, and the purified protein was unable to transfer biotin from enzyme-bound biotinoyl-adenylate either to the natural acceptor protein or to a biotin-accepting peptide sequence. Consistent with the transcriptional repression data, each of the purified mutant proteins showed increased affinity for the biotin operator DNA in electrophoretic mobility shift assays. Surprisingly, although most of the mutations were located in the catalytic domain, all of those tested, except G154D BirA, had normal ligase activity. Most of the mutations that gave superrepressor phenotypes altered residues located close to the dimerization interface of BirA. However, two mutations were located at sites well removed from the interface. The properties of the superrepressor mutants strengthen and extend other data indicating that BirA function entails extensive interactions among the three domains of the protein and show that normal ligase activity does not ensure normal DNA binding.
Figures







References
-
- Barker DF, Campbell AM. 1981. The birA gene of Escherichia coli encodes a biotin holoenzyme synthetase. J. Mol. Biol. 146:451–467 - PubMed
-
- Barker DF, Campbell AM. 1981. Genetic and biochemical characterization of the birA gene and its product: evidence for a direct role of biotin holoenzyme synthetase in repression of the biotin operon in Escherichia coli. J. Mol. Biol. 146:469–492 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

