β2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein
- PMID: 22210825
- PMCID: PMC3262659
- DOI: 10.1165/rcmb.2011-0217OC
β2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein
Abstract
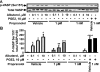
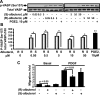
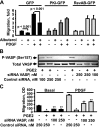
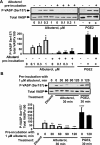
Severe asthma manifests as airway remodeling and irreversible airway obstruction, in part because of the proliferation and migration of human airway smooth muscle (HASM) cells. We previously reported that cyclic adenosine monophosphate-mobilizing agents, including β(2)-adrenergic receptor (β(2)AR) agonists, which are mainstay of asthma therapy, and prostaglandin E2 (PGE2), inhibit the migration of HASM cells, although the mechanism for this migration remains unknown. Vasodilator-stimulated phosphoprotein (VASP), an anticapping protein, modulates the formation of actin stress fibers during cell motility, and is negatively regulated by protein kinase A (PKA)-specific inhibitory phosphorylation at serine 157 (Ser157). Here, we show that treatment with β(2)AR agonists and PGE2 induces the PKA-dependent phosphorylation of VASP and inhibits the migration of HASM cells. The stable expression of PKA inhibitory peptide and the small interfering (si) RNA-induced depletion of VASP abolish the inhibitory effects of albuterol and PGE2 on the migration of HASM cells. Importantly, prolonged treatment with albuterol prevents the agonist-induced phosphorylation of VASP at Ser157, and reverses the inhibitory effects of albuterol and formoterol, but not PGE2, on the basal and PDGF-induced migration of HASM cells. Collectively, our data demonstrate that β(2)AR agonists selectively inhibit the migration of HASM cells via a β(2)AR/PKA/VASP signaling pathway, and that prolonged treatment with albuterol abolishes the inhibitory effect of β-agonists on the phosphorylation of VASP and migration of HASM cells because of β(2)AR desensitization.
Figures


References
-
- Joubert P, Hamid Q. Role of airway smooth muscle in airway remodeling. J Allergy Clin Immunol 2005;116:713–716 - PubMed
-
- Gizycki MJ, Adelroth E, Rogers AV, O'Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol 1997;16:664–673 - PubMed
-
- Carlin SM, Roth M, Black JL. Urokinase potentiates PDGF-induced chemotaxis of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2003;284:L1020–L1026 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

