Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea
- PMID: 22210909
- PMCID: PMC3292873
- DOI: 10.4049/jimmunol.1101968
Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea
Abstract
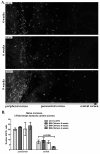
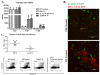
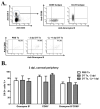
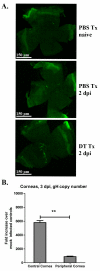
Dendritic cells (DCs) regulate both innate and adaptive immune responses. In this article, we exploit the unique avascularity of the cornea to examine a role for local or very early infiltrating DCs in regulating the migration of blood-derived innate immune cells toward HSV-1 lesions. A single systemic diphtheria toxin treatment 2 d before HSV-1 corneal infection transiently depleted CD11c(+) DCs from both the cornea and lymphoid organs of CD11c-DTR bone marrow chimeric mice for up to 24 h postinfection. Transient DC depletion significantly delayed HSV-1 clearance from the cornea through 6 d postinfection. No further compromise of viral clearance was observed when DCs were continuously depleted throughout the first week of infection. DC depletion did not influence extravasation of NK cells, inflammatory monocytes, or neutrophils into the peripheral cornea, but it did significantly reduce migration of NK cells and inflammatory monocytes, but not neutrophils, toward the HSV-1 lesion in the central cornea. Depletion of NK cells resulted in similar loss of viral control to transient DC ablation. Our findings demonstrate that resident corneal DCs and/or those that infiltrate the cornea during the first 24 h after HSV-1 infection contribute to the migration of NK cells and inflammatory monocytes into the central cornea, and are consistent with a role for NK cells and possibly inflammatory monocytes, but not polymorphonuclear neutrophils, in clearing HSV-1 from the infected cornea.
Figures



Similar articles
-
PD-L1/B7-H1 Inhibits Viral Clearance by Macrophages in HSV-1-Infected Corneas.J Immunol. 2018 Jun 1;200(11):3711-3719. doi: 10.4049/jimmunol.1700417. Epub 2018 Apr 18. J Immunol. 2018. PMID: 29669784 Free PMC article.
-
Cornea-infiltrating and lymph node dendritic cells contribute to CD4+ T cell expansion after herpes simplex virus-1 ocular infection.J Immunol. 2015 Jan 1;194(1):379-87. doi: 10.4049/jimmunol.1402326. Epub 2014 Nov 24. J Immunol. 2015. PMID: 25422507 Free PMC article.
-
Dual TLR2/9 Recognition of Herpes Simplex Virus Infection Is Required for Recruitment and Activation of Monocytes and NK Cells and Restriction of Viral Dissemination to the Central Nervous System.Front Immunol. 2018 Apr 30;9:905. doi: 10.3389/fimmu.2018.00905. eCollection 2018. Front Immunol. 2018. PMID: 29760708 Free PMC article.
-
Type I interferon and lymphangiogenesis in the HSV-1 infected cornea - are they beneficial to the host?Prog Retin Eye Res. 2013 Sep;36:281-91. doi: 10.1016/j.preteyeres.2013.06.003. Epub 2013 Jul 19. Prog Retin Eye Res. 2013. PMID: 23876483 Free PMC article. Review.
-
NK/DC crosstalk in anti-viral response.Adv Exp Med Biol. 2012;946:295-308. doi: 10.1007/978-1-4614-0106-3_17. Adv Exp Med Biol. 2012. PMID: 21948375 Review.
Cited by
-
Antigen-presenting cells are stratified within normal human corneas and are rapidly mobilized during ex vivo viral infection.Invest Ophthalmol Vis Sci. 2014 Feb 24;55(2):1118-23. doi: 10.1167/iovs.13-13523. Invest Ophthalmol Vis Sci. 2014. PMID: 24508792 Free PMC article.
-
Herpes simplex virus-1 KOS-63 strain is virulent and causes titer-dependent corneal nerve damage and keratitis.Sci Rep. 2021 Feb 19;11(1):4267. doi: 10.1038/s41598-021-83412-9. Sci Rep. 2021. PMID: 33608598 Free PMC article.
-
Immunological Control of Herpes Simplex Virus Type 1 Infection: A Non-Thermal Plasma-Based Approach.Viruses. 2025 Apr 23;17(5):600. doi: 10.3390/v17050600. Viruses. 2025. PMID: 40431612 Free PMC article. Review.
-
The Host-Pathogen Interplay: A Tale of Two Stories within the Cornea and Posterior Segment.Microorganisms. 2023 Aug 12;11(8):2074. doi: 10.3390/microorganisms11082074. Microorganisms. 2023. PMID: 37630634 Free PMC article. Review.
-
Cortisol biosynthesis in the human ocular surface innate immune response.PLoS One. 2014 Apr 15;9(4):e94913. doi: 10.1371/journal.pone.0094913. eCollection 2014. PLoS One. 2014. PMID: 24736562 Free PMC article.
References
-
- Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001;22:78–83. - PubMed
-
- Kaisho T, Akira S. Toll-like receptors and their signaling mechanism in innate immunity. Acta Odontol. Scand. 2001;59:124–130. - PubMed
-
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. - PubMed
-
- Eidsmo L, Allan R, Caminschi I, van Rooijen N, Heath WR, Carbone FR. Differential migration of epidermal and dermal dendritic cells during skin infection. J. Immunol. 2009;182:3165–3172. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

