MRG-1 is required for genomic integrity in Caenorhabditis elegans germ cells
- PMID: 22212480
- PMCID: PMC3343660
- DOI: 10.1038/cr.2012.2
MRG-1 is required for genomic integrity in Caenorhabditis elegans germ cells
Abstract
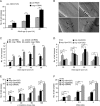
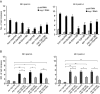
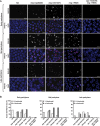
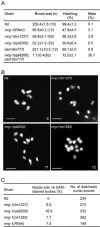
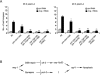
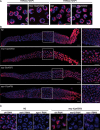
During meiotic cell division, proper chromosome synapsis and accurate repair of DNA double strand breaks (DSBs) are required to maintain genomic integrity, loss of which leads to apoptosis or meiotic defects. The mechanisms underlying meiotic chromosome synapsis, DSB repair and apoptosis are not fully understood. Here, we report that the chromodomain-containing protein MRG-1 is an important factor for genomic integrity in meiosis in Caenorhabditis elegans. Loss of mrg-1 function resulted in a significant increase in germ cell apoptosis that was partially inhibited by mutations affecting DNA damage checkpoint genes. Consistently, mrg-1 mutant germ lines exhibited SPO-11-generated DSBs and elevated exogenous DNA damage-induced chromosome fragmentation at diakinesis. In addition, the excessive apoptosis in mrg-1 mutants was partially suppressed by loss of the synapsis checkpoint gene pch-2, and a significant number of meiotic nuclei accumulated at the leptotene/zygotene stages with an elevated level of H3K9me2 on the chromatin, which was similarly observed in mutants deficient in the synaptonemal complex, suggesting that the proper progression of chromosome synapsis is likely impaired in the absence of mrg-1. Altogether, these findings suggest that MRG-1 is critical for genomic integrity by promoting meiotic DSB repair and synapsis progression in meiosis.
Figures



Similar articles
-
A Defective Meiotic Outcome of a Failure in Homologous Pairing and Synapsis Is Masked by Meiotic Quality Control.PLoS One. 2015 Aug 6;10(8):e0134871. doi: 10.1371/journal.pone.0134871. eCollection 2015. PLoS One. 2015. PMID: 26247362 Free PMC article.
-
Maintenance of Genome Integrity by Mi2 Homologs CHD-3 and LET-418 in Caenorhabditis elegans.Genetics. 2018 Mar;208(3):991-1007. doi: 10.1534/genetics.118.300686. Epub 2018 Jan 16. Genetics. 2018. PMID: 29339410 Free PMC article.
-
C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression.PLoS Genet. 2007 Nov;3(11):e191. doi: 10.1371/journal.pgen.0030191. PLoS Genet. 2007. PMID: 17983271 Free PMC article.
-
Methods for studying the DNA damage response in the Caenorhabdatis elegans germ line.Methods Cell Biol. 2012;107:321-52. doi: 10.1016/B978-0-12-394620-1.00011-4. Methods Cell Biol. 2012. PMID: 22226529 Review.
-
Mechanisms of germ cell survival and plasticity in Caenorhabditis elegans.Biochem Soc Trans. 2022 Oct 31;50(5):1517-1526. doi: 10.1042/BST20220878. Biochem Soc Trans. 2022. PMID: 36196981 Free PMC article. Review.
Cited by
-
Programmed cell death and clearance of cell corpses in Caenorhabditis elegans.Cell Mol Life Sci. 2016 Jun;73(11-12):2221-36. doi: 10.1007/s00018-016-2196-z. Epub 2016 Apr 5. Cell Mol Life Sci. 2016. PMID: 27048817 Free PMC article. Review.
-
Proteasome regulation of the chromodomain protein MRG-1 controls the balance between proliferative fate and differentiation in the C. elegans germ line.Development. 2015 Jan 15;142(2):291-302. doi: 10.1242/dev.115147. Development. 2015. PMID: 25564623 Free PMC article.
-
A Defective Meiotic Outcome of a Failure in Homologous Pairing and Synapsis Is Masked by Meiotic Quality Control.PLoS One. 2015 Aug 6;10(8):e0134871. doi: 10.1371/journal.pone.0134871. eCollection 2015. PLoS One. 2015. PMID: 26247362 Free PMC article.
-
Histone methyltransferases MES-4 and MET-1 promote meiotic checkpoint activation in Caenorhabditis elegans.PLoS Genet. 2012;8(11):e1003089. doi: 10.1371/journal.pgen.1003089. Epub 2012 Nov 15. PLoS Genet. 2012. PMID: 23166523 Free PMC article.
-
Maintenance of Genome Integrity by Mi2 Homologs CHD-3 and LET-418 in Caenorhabditis elegans.Genetics. 2018 Mar;208(3):991-1007. doi: 10.1534/genetics.118.300686. Epub 2018 Jan 16. Genetics. 2018. PMID: 29339410 Free PMC article.
References
-
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Garcia-Muse T, Boulton SJ. Meiotic recombination in Caenorhabditis elegans. Chromosome Res. 2007;15:607–621. - PubMed
-
- Colaiacovo MP. The many facets of SC function during C. elegans meiosis. Chromosoma. 2006;115:195–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

