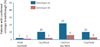The role of ribavirin in direct acting antiviral drug regimens for chronic hepatitis C
- PMID: 22212579
- PMCID: PMC3682505
- DOI: 10.1111/j.1478-3231.2011.02711.x
The role of ribavirin in direct acting antiviral drug regimens for chronic hepatitis C
Abstract
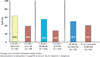
Despite years of clinical use and extensive research efforts, the mechanism of action of ribavirin (RBV) is not well understood. Although it has only a mild, transient antiviral effect on HCV replication when administered as monotherapy, when combined with interferon, RBV improves sustained virological response (SVR) rates by approximately 25-30%. Proposed mechanisms of action for RBV against HCV include (1) a direct effect against the HCV RNA dependent RNA polymerase; (2) induction of misincorporation of nucleotides leading to lethal mutagenesis; (3) depletion of intracellular pools via inhibition of inosine monophosphate dehydrogenase; (4) alteration in the cytokine balance between a Th2 profile (anti-inflammatory) to a Th1 profile (pro-inflammatory); and (5) potentiating the effect of interferon via up-regulation of genes involved in interferon signalling. Given the lack of a clear understanding of RBV mechanism of action, it has been challenging to confidently position this drug with new direct antiviral agents (DAA). However, early clinical studies provide strong evidence for a benefit of RBV in combination with DAAs for both IFN containing and sparing regimens. The addition of RBV reduces viral breakthroughs and/or relapses, at least when drugs with low to moderate genetic barriers to resistance are paired together. This is particularly true in patients harbouring HCV subtype 1a. Ongoing studies are now addressing the utility of RBV in nucleoside containing DAA regimens, which offer both potent antiviral activity as well as a high genetic barrier to resistance. It is remarkable that the age-old question of the role of RBV in the future of HCV therapy remains as real today as it was two decades ago.
© 2012 John Wiley & Sons A/S.
Conflict of interest statement
Figures
Similar articles
-
Autoantibodies to cytoplasmic rods and rings in patients with hepatitis C virus infection treated with direct-acting antivirals: The role of prior treatment with interferon plus ribavirin.Gastroenterol Hepatol. 2019 Feb;42(2):82-89. doi: 10.1016/j.gastrohep.2018.08.005. Epub 2018 Nov 13. Gastroenterol Hepatol. 2019. PMID: 30446175 English, Spanish.
-
Ribavirin induced hemolysis: a novel mechanism of action against chronic hepatitis C virus infection.World J Gastroenterol. 2014 Nov 21;20(43):16184-90. doi: 10.3748/wjg.v20.i43.16184. World J Gastroenterol. 2014. PMID: 25473172 Free PMC article. Review.
-
Phase III results of Boceprevir in treatment naïve patients with chronic hepatitis C genotype 1.Liver Int. 2012 Feb;32 Suppl 1:27-31. doi: 10.1111/j.1478-3231.2011.02725.x. Liver Int. 2012. PMID: 22212568 Review.
-
Monitoring virological responses to interferon-ribavirin and interferon monotherapy of chronic hepatitis C re-treated due to relapse or non-response.Scand J Infect Dis. 2001;33(2):110-5. doi: 10.1080/003655401750065481. Scand J Infect Dis. 2001. PMID: 11233844
-
All Oral Interferon-free Direct-acting Antivirals as Combination Therapies to Cure Hepatitis C.Curr Mol Med. 2018;18(7):409-435. doi: 10.2174/1566524019666190104110439. Curr Mol Med. 2018. PMID: 30608042 Review.
Cited by
-
Ribavirin exerts differential effects on functions of Cd4+ Th1, Th2, and regulatory T cell clones in hepatitis C.PLoS One. 2012;7(7):e42094. doi: 10.1371/journal.pone.0042094. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848715 Free PMC article.
-
Increased baseline proinflammatory cytokine production in chronic hepatitis C patients with rapid virological response to peginterferon plus ribavirin.PLoS One. 2013 Jul 9;8(7):e67770. doi: 10.1371/journal.pone.0067770. Print 2013. PLoS One. 2013. PMID: 23874444 Free PMC article.
-
Therapeutic effect of intravenous acyclovir in children with infectious mononucleosis and immune function.Am J Transl Res. 2023 Aug 15;15(8):5258-5266. eCollection 2023. Am J Transl Res. 2023. PMID: 37692931 Free PMC article.
-
The impact of ribavirin on real-world adherence rates in hepatitis C patients treated with sofosbuvir plus simeprevir.Clinicoecon Outcomes Res. 2015 Dec 17;7:637-42. doi: 10.2147/CEOR.S87261. eCollection 2015. Clinicoecon Outcomes Res. 2015. PMID: 26719716 Free PMC article.
-
The application and mechanism of action of ribavirin in therapy of hepatitis C.Antivir Chem Chemother. 2012 Sep 25;23(1):1-12. doi: 10.3851/IMP2125. Antivir Chem Chemother. 2012. PMID: 22592135 Free PMC article. Review.
References
-
- Brillanti S, Garson J, Foli M, et al. A pilot study of combination therapy with ribavirin plus interferon alpha for interferon alpha resistant chronic hepatitis C. Gatsroenterology. 1994;107:812–819. - PubMed
-
- Reichard O, Norkrans G, Fryden A, et al. Randomised, double-blind, placebo-controlled trial of interferon alpha2b with and without ribavirin for chronic heptitis C. Lancet. 1998;351:83–87. - PubMed
-
- McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. - PubMed
-
- Pawlotsky JM, Dahari H, Neumann AU, et al. Antiviral action of ribavirin in chronic hepatitis C. Gastroenterology. 2004;126:703–714. - PubMed
-
- Bronowicki JP, Ouzan D, Asselah T, et al. Effect of ribavirin in genotype 1 patients with hepatitis C responding to pegylated interferon alfa-2a plus ribavirin. Gastroenterology. 2006;131:1040–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources