Deficiency of DNA repair nuclease ERCC1-XPF promotes prostate cancer progression in a tissue recombination model
- PMID: 22212909
- PMCID: PMC3490419
- DOI: 10.1002/pros.22472
Deficiency of DNA repair nuclease ERCC1-XPF promotes prostate cancer progression in a tissue recombination model
Abstract
Background: The excision repair cross complementing (ERCC1) gene product plays a vital role in the nucleotide excision repair (NER) and DNA interstrand crosslink repair pathways, which protect the genome from mutations and chromosomal aberrations, respectively. Genetic deletion of Ercc1 in the mouse causes dramatically accelerated aging. We examined the effect of Ercc1 deletion in the development of prostate cancer in a prostate recapitulation model as Ercc1 deficient mice die within four weeks of birth.
Methods: Prostate tissues from Ercc1(-/-) mice or wild-type littermates were combined with embryonic rat urogenital mesenchyme and grown as renal grafts for a total of 8, 16, and 24 weeks before histological, expression and proliferative evaluation.
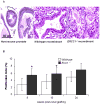
Results: Invasive adenocarcinoma was observed in Ercc1(-/-) tissue recombinants but not wild-type as early as 8 weeks post-grafting. PIN-like lesions in Ercc1(-/-) tissue recombinants had more cytologic and architectural atypia than wild-type (P = 0.02, P = 0.0065, and P = 0.0003 at the 8, 16, and 24 weeks, respectively), as well as more proliferative cells (P = 0.022 and P = 0.033 at 8 and 16 weeks, respectively). With serial grafting, Ercc1(-/-) tissue recombinants progressed to a more severe histopathological phenotype more rapidly than wild-type (P = 0.011).
Conclusions: Results show that ERCC1 and by implication the NER and/or interstrand crosslink repair mechanisms protect against prostate carcinogenesis and mutations or polymorphisms affecting these DNA repair pathways may predispose prostate epithelial cells to transformation.
Copyright © 2011 Wiley Periodicals, Inc.
Conflict of interest statement
Disclosure Statement: The authors declare that they have no affiliations with any organization that may have a direct interest in the research described, or a real or perceived conflict of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures




References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61:6025–6028. - PubMed
-
- Isaacs WB, Bova GS, Morton RA, Bussemakers MJ, Brooks JD, Ewing CM. Molecular biology of prostate cancer. Semin Oncol. 1994;21:514–521. - PubMed
-
- Coughlin SS, Hall IJ. A review of genetic polymorphisms and prostate cancer risk. Ann Epidemiol. 2002;12:182–196. - PubMed
-
- Dong JT. Prevalent mutations in prostate cancer. J Cell Biochem. 2006;97:433–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

