One thousand patients with primary myelofibrosis: the mayo clinic experience
- PMID: 22212965
- PMCID: PMC3538387
- DOI: 10.1016/j.mayocp.2011.11.001
One thousand patients with primary myelofibrosis: the mayo clinic experience
Abstract
Objective: To share our decades of experience with primary myelofibrosis and underscore the importance of outcomes research studies in designing clinical trials and interpreting their results.
Patients and methods: One thousand consecutive patients with primary myelofibrosis seen at Mayo Clinic between November 4, 1977, and September 1, 2011, were considered. The International Prognostic Scoring System (IPSS), dynamic IPSS (DIPSS), and DIPSS-plus were applied for risk stratification. Separate analyses were included for patients seen at time of referral (N=1000), at initial diagnosis (N=340), and within or after 1 year of diagnosis (N=660).
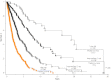
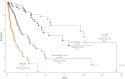
Results: To date, 592 deaths and 68 leukemic transformations have been documented. Parameters at initial diagnosis vs time of referral included median age (66 vs 65 years), male sex (61% vs 62%), red cell transfusion need (24% vs 38%), hemoglobin level less than 10 g/dL (38% vs 54%), platelet count less than 100 × 10(9)/L (18% vs 26%), leukocyte count more than 25 × 10(9)/L (13% vs 16%), marked splenomegaly (21% vs 31%), constitutional symptoms (29% vs 34%), and abnormal karyotype (31% vs 41%). Mutational frequencies were 61% for JAK2V617F, 8% for MPLW515, and 4% for IDH1/2. DIPSS-plus risk distributions at time of referral were 10% low, 15% intermediate-1, 37% intermediate-2, and 37% high. The corresponding median survivals were 17.5, 7.8, 3.6, and 1.8 years vs 20.0, 14.3, 5.3, and 1.7 years for patients younger than 60 years of age. Compared with both DIPSS and IPSS, DIPSS-plus showed better discrimination among risk groups. Five-year leukemic transformation rates were 6% and 21% in low- and high-risk patients, respectively.
Conclusion: The current document should serve as a valuable resource for patients and physicians and provides context for the design and interpretation of clinical trials.
Copyright © 2012 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Vardiman J.W., Thiele J., Arber D.A. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. - PubMed
-
- Barosi G., Mesa R.A., Thiele J. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–438. - PubMed
-
- Heuck G. Zwei Falle von Leukamie mit eigenthumlichem Blut- resp. Knochenmarksbefund. [Two cases of leukemia with peculiar blood and bone marrow findings, respectively]Arch Pathol Anat Physiol Virchows. 1879;78:475–496.
-
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–375. - PubMed
-
- Jacobson R.J., Salo A., Fialkow P.J. Agnogenic myeloid metaplasia: a clonal proliferation of hematopoietic stem cells with secondary myelofibrosis. Blood. 1978;51(2):189–194. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

