VE-cadherin trans-interactions modulate Rac activation and enhancement of lung endothelial barrier by iloprost
- PMID: 22213015
- PMCID: PMC3328640
- DOI: 10.1002/jcp.24041
VE-cadherin trans-interactions modulate Rac activation and enhancement of lung endothelial barrier by iloprost
Abstract
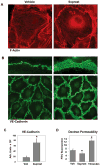
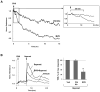
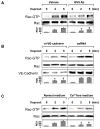
Small GTPase Rac is important regulator of endothelial cell (EC) barrier enhancement by prostacyclin characterized by increased peripheral actin cytoskeleton and increased interactions between VE-cadherin and other adherens junction (AJ) proteins. This study utilized complementary approaches including siRNA knockdown, culturing in Ca(2+) -free medium, and VE-cadherin blocking antibody to alter VE-cadherin extracellular interactions to investigate the role of VE-cadherin outside-in signaling in modulation of Rac activation and EC barrier regulation by prostacyclin analog iloprost. Spatial analysis of Rac activation in pulmonary EC by FRET revealed additional spike in iloprost-induced Rac activity at the sites of newly formed cell-cell junctions. In contrast, disruption of VE-cadherin extracellular trans-interactions suppressed iloprost-activated Rac signaling and attenuated EC barrier enhancement and cytoskeletal remodeling. These inhibitory effects were associated with decreased membrane accumulation and activation of Rac-specific guanine nucleotide exchange factors (GEFs) Tiam1 and Vav2. Conversely, plating of pulmonary EC on surfaces coated with extracellular VE-cadherin domain further promoted iloprost-induced Rac signaling. In the model of thrombin-induced EC barrier recovery, blocking of VE-cadherin trans-interactions attenuated activation of Rac pathway during recovery phase and delayed suppression of Rho signaling and restoration of EC barrier properties. These results suggest that VE-cadherin outside-in signaling controls locally Rac activity stimulated by barrier protective agonists. This control is essential for maximal EC barrier enhancement and accelerated barrier recovery.
Copyright © 2011 Wiley Periodicals, Inc.
Figures








References
-
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J Cell Sci. 2006;119(Pt 5):866–875. - PubMed
-
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. - PubMed
-
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

