Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations
- PMID: 22213121
- PMCID: PMC4147853
- DOI: 10.1002/jcb.24046
Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations
Abstract
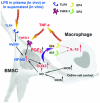
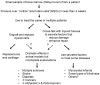
There is currently great interest in the use of mesenchymal stem/stromal cells (MSCs) for the therapy of many diseases of animals and humans. However, we are still left with the serious challenges in explaining the beneficial effects of the cells. Hence, it is essential to work backward from dramatic results obtained in vivo to the cellular and molecular explanations in order to discover the secrets of MSCs. This review will focus on recent data that have changed the paradigms for understanding the therapeutic potentials of MSCs.
Copyright © 2011 Wiley Periodicals, Inc.
Figures





References
-
- Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011;10:410–415. - PubMed
-
- Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. - PubMed
-
- Bianco P, Sacchetti B, Riminucci M. Osteoprogenitors and the hematopoietic microenvironment. Best Pract Res Clin Haematol. 2011;24:37–47. - PubMed
-
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

