Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filaria-malaria co-infection
- PMID: 22213332
- PMCID: PMC3430845
- DOI: 10.1002/eji.201141991
Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filaria-malaria co-infection
Abstract
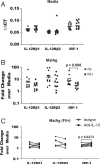
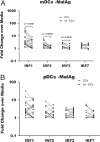
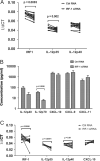
In areas where polyparasitism is highly prevalent, the impact of multiple parasites on the host response is underestimated. In particular, the presence of helminth infection coincident with malaria profoundly alters the production of malaria-specific IFN-γ, IL-12p70, CXCL9, CXCL10 and CXCL11, cytokines/chemokines known to be critical in mediating malaria-specific immunity. In order to elucidate the mechanisms underlying the suppression of malaria-specific cytokines/chemokines, we assessed the expression of malaria-specific IL-12Rβ1, IL-12Rβ2 and interferon regulatory factor (IRF)-1 in blood obtained from 18 filaria-infected (Fil(+)) and 17 filaria-uninfected (Fil(-)) individuals in a filaria-malaria co-endemic region of Mali. We found that Fil(+) individuals had significantly lower RNA expression of IRF-1 but not IL-12Rβ1 or IL-12Rβ2 in response to malaria antigen stimulation. We also measured the frequency of IL-12-producing DCs from these subjects and found that Fil(+) subjects had lower frequencies of IL-12(+) mDCs after malaria antigen stimulation than did the Fil(-) subjects. Modeling these data in vitro, we found that mDCs pre-exposed to live microfilariae not only produced significantly lower levels of CXCL-9, CXCL-10, IL-12p35, IL-12p40, IL-12p19 and CXCL-11 following stimulation with malaria antigen but also markedly downregulated the expression of IRF-1, IRF-2 and IRF-3 compared with microfilaria-unexposed mDCs. siRNA-inhibition of irf-1 in mDCs downregulated the production of IL-12p70 through repression of IL-12p35. Our data demonstrate that the modulation of IRFs seen in filarial (and presumably other tissue-invasive helminths) infection underlies the suppression of malaria-specific cytokines/chemokines that play a crucial role in immunity to malaria.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- WHO . World Malaria Report 2009. Geneve: 2010. http://www.who.int/malariaworld_malaria_report_2009/en.index.html.
-
- Le Hesran JY, Akiana J, Ndiaye el HM, Dia M, Senghor P, Konate L. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans. R. Soc. Trop. Med. Hyg. 2004;98:397–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

