A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment
- PMID: 22213571
- PMCID: PMC3987771
- DOI: 10.1002/cyto.a.22012
A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment
Abstract
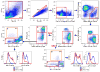
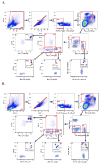
Currently, there is no standardized panel for immunophenotyping myeloid cells in mouse spleen using flow cytometry. Markers such as CD11b, CD11c, F4/80, Gr-1, Ly6C, and Ly6G have long been used to identify various splenic cell myeloid populations. Flow cytometry and fluorescence-activated cell sorting (FACS) analysis demonstrated that Ly6G/Ly6C markers are superior to Gr-1 for identifying splenic neutrophils, eosinophils, and subsets of monocytes/macrophages. Moreover, these experiments showed that F4/80 is not required for identifying these myeloid subsets and that many of the commercially available preparations of anti-F4/80 antibodies stain poorly for this antigen in spleen. Taken together, we have now developed an informative flow cytometry panel that can be combined with other cell markers to further delineate subpopulations of mouse splenic myeloid cells. This panel will be highly useful to investigators in the flow cytometry field, as there is a critical need to standardize the analysis of myeloid cell subsets.
Copyright © 2011 International Society for Advancement of Cytometry.
Figures


References
-
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. - PubMed
-
- Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Current Opinion in Hematology. 2010;17:53–9. - PubMed
-
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. European Journal of Immunology. 1981;11:805–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

