dEHBP1 controls exocytosis and recycling of Delta during asymmetric divisions
- PMID: 22213802
- PMCID: PMC3255984
- DOI: 10.1083/jcb.201106088
dEHBP1 controls exocytosis and recycling of Delta during asymmetric divisions
Abstract
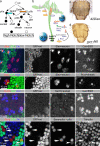
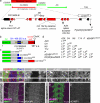
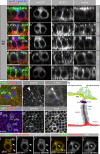
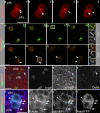
Notch signaling governs binary cell fate determination in asymmetrically dividing cells. Through a forward genetic screen we identified the fly homologue of Eps15 homology domain containing protein-binding protein 1 (dEHBP1) as a novel regulator of Notch signaling in asymmetrically dividing cells. dEHBP1 is enriched basally and at the actin-rich interface of pII cells of the external mechanosensory organs, where Notch signaling occurs. Loss of function of dEHBP1 leads to up-regulation of Sanpodo, a regulator of Notch signaling, and aberrant trafficking of the Notch ligand, Delta. Furthermore, Sec15 and Rab11, which have been previously shown to regulate the localization of Delta, physically interact with dEHBP1. We propose that dEHBP1 functions as an adaptor molecule for the exocytosis and recycling of Delta, thereby affecting cell fate decisions in asymmetrically dividing cells.
Figures



Similar articles
-
Endocytosis and control of Notch signaling.Curr Opin Cell Biol. 2012 Aug;24(4):534-40. doi: 10.1016/j.ceb.2012.06.006. Epub 2012 Jul 18. Curr Opin Cell Biol. 2012. PMID: 22818956 Free PMC article. Review.
-
Drosophila EHBP1 regulates Scabrous secretion during Notch-mediated lateral inhibition.J Cell Sci. 2013 Aug 15;126(Pt 16):3686-96. doi: 10.1242/jcs.126292. Epub 2013 Jun 20. J Cell Sci. 2013. PMID: 23788431 Free PMC article.
-
Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors.Dev Cell. 2005 Sep;9(3):351-63. doi: 10.1016/j.devcel.2005.06.010. Dev Cell. 2005. PMID: 16137928
-
Phosphatidic acid increases Notch signalling by affecting Sanpodo trafficking during Drosophila sensory organ development.Sci Rep. 2020 Dec 10;10(1):21731. doi: 10.1038/s41598-020-78831-z. Sci Rep. 2020. PMID: 33303974 Free PMC article.
-
Robust selection of sensory organ precursors by the Notch-Delta pathway.Curr Opin Cell Biol. 2011 Dec;23(6):663-7. doi: 10.1016/j.ceb.2011.09.005. Epub 2011 Sep 29. Curr Opin Cell Biol. 2011. PMID: 21963301 Review.
Cited by
-
Endocytosis and control of Notch signaling.Curr Opin Cell Biol. 2012 Aug;24(4):534-40. doi: 10.1016/j.ceb.2012.06.006. Epub 2012 Jul 18. Curr Opin Cell Biol. 2012. PMID: 22818956 Free PMC article. Review.
-
Non-cell autonomous regulation of cell-cell signaling and differentiation by mitochondrial ROS.J Cell Biol. 2024 Dec 2;223(12):e202401084. doi: 10.1083/jcb.202401084. Epub 2024 Nov 13. J Cell Biol. 2024. PMID: 39535785 Free PMC article.
-
Spatio-Temporal Regulation of Notch Activation in Asymmetrically Dividing Sensory Organ Precursor Cells in Drosophila melanogaster Epithelium.Cells. 2024 Jun 30;13(13):1133. doi: 10.3390/cells13131133. Cells. 2024. PMID: 38994985 Free PMC article. Review.
-
Drosophila as a Model for Infectious Diseases.Int J Mol Sci. 2021 Mar 8;22(5):2724. doi: 10.3390/ijms22052724. Int J Mol Sci. 2021. PMID: 33800390 Free PMC article. Review.
-
RAB-10 Promotes EHBP-1 Bridging of Filamentous Actin and Tubular Recycling Endosomes.PLoS Genet. 2016 Jun 6;12(6):e1006093. doi: 10.1371/journal.pgen.1006093. eCollection 2016 Jun. PLoS Genet. 2016. PMID: 27272733 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

