Remodelling of the natural product fumagillol employing a reaction discovery approach
- PMID: 22213919
- PMCID: PMC3254213
- DOI: 10.1038/nchem.1178
Remodelling of the natural product fumagillol employing a reaction discovery approach
Abstract
In the search for new biologically active molecules, diversity-oriented synthetic strategies break through the limitation of traditional library synthesis by sampling new chemical space. Many natural products can be regarded as intriguing starting points for diversity-oriented synthesis, wherein stereochemically rich core structures may be reorganized into chemotypes that are distinctly different from the parent structure. Ideally, to be suited to library applications, such transformations should be general and involve few steps. With this objective in mind, the highly oxygenated natural product fumagillol has been successfully remodelled in several ways using a reaction-discovery-based approach. In reactions with amines, excellent regiocontrol in a bis-epoxide opening/cyclization sequence can be obtained by size-dependent interaction of an appropriate catalyst with the parent molecule, forming either perhydroisoindole or perhydroisoquinoline products. Perhydroisoindoles can be further remodelled by cascade processes to afford either morpholinone or bridged 4,1-benzoxazepine-containing structures.
Conflict of interest statement
The authors declare no competing financial interests. Supplementary information and chemical compound information accompany this paper at
Figures
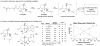

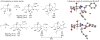
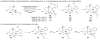
References
-
- Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical Success. J Med Chem. 2009;52:6752–6756. - PubMed
-
- Galloway WRJD, Isidro-Llobet A, Spring DR. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat Commun 1. 2010;80:1–13. - PubMed
-
- Mang C, Jakupovic S, Schunk S, Ambrosi H-D, Schwarz O, Jakupovic J. Natural products in combinatorial chemistry: an andrographolide-based library. J Comb Chem. 2006;8:268–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

