Site- and enantioselective formation of allene-bearing tertiary or quaternary carbon stereogenic centers through NHC-Cu-catalyzed allylic substitution
- PMID: 22214185
- PMCID: PMC3267425
- DOI: 10.1021/ja211269w
Site- and enantioselective formation of allene-bearing tertiary or quaternary carbon stereogenic centers through NHC-Cu-catalyzed allylic substitution
Abstract
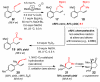
Catalytic enantioselective allylic substitutions that result in addition of an allenyl group (<2% propargyl addition) and formation of tertiary or quaternary C-C bonds are described. Commercially available allenylboronic acid pinacol ester is used. Reactions are promoted by a 5.0-10 mol % loading of sulfonate-bearing chiral bidentate N-heterocyclic carbene (NHC) complexes of copper, which exhibit the unique ability to furnish chiral products arising from the S(N)2' mode of addition. Allenyl-containing products are generated in up to 95% yield, >98% S(N)2' selectivity, and 99:1 enantiomeric ratio (er). Site-selective NHC-Cu-catalyzed hydroboration of enantiomerically enriched allenes and conversion to the corresponding β-vinyl ketones demonstrates the method's utility.
© 2012 American Chemical Society
Figures

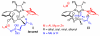
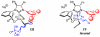

References
-
-
Krause N, Hashmi ASK, editors. Modern Allene Chemistry. Wiley–VCH; Weinheim, Germany: 2004. Yu S, Ma S. Chem. Commun. 2011:5384. For allene-containing natural products, see: Hoffmann-Röder A, Krause N. Angew. Chem., Int. Ed. 2004;43:1196.
-
-
-
For catalytic enantioselective allene addition to aldehydes, see: Yu C-M, Yoon S-K, Baek K, Lee J-Y. Angew. Chem., Int. Ed. 1998;37:2392. Inoue M, Nakada M. Angew. Chem., Int. Ed. 2006;45:252. Xia G, Yamamoto H. J. Am. Chem. Soc. 2007;129:496. Coeffard V, Aylward M, Guiry P. J. Angew. Chem., Int. Ed. 2009;48:9152. Durán-Galván M, Worlikar SA, Connell BT. Tetrahedron. 2010;66:7707. For enantioselective synthesis of allenes by other catalytic approaches, see: Imada Y, Nishida M, Kutsuwa K, Murahashi S-I, Naota T. Org. Lett. 2005;7:5837. Zhang W, Zheng S, Liu N, Werness JB, Guzei IA, Tang W. J. Am. Chem. Soc. 2010;132:3664.
-
-
-
For reviews on catalytic allylic alkylations with “hard” C-based nucleophilic reagents, see: Hoveyda AH, Hird AW, Kacprzynski MA. Chem. Commun. 2004:1779. Yorimitsu H, Oshima K. Angew. Chem., Int. Ed. 2005;44:4435. Alexakis A, Bäckvall JE, Krause N, Pàmies O, Diéguez M. Chem. Rev. 2008;108:2796.
-
-
-
For examples with amino acid-based chiral Cu complexes, see: Murphy KE, Hoveyda AH. J. Am. Chem. Soc. 2003;125:4690. Kacprzynski MA, Hoveyda AH. J. Am. Chem. Soc. 2004;126:10676. For examples with NHC–Cu complexes see: Larsen AO, Leu W, Oberhuber CN, Campbell JE, Hoveyda AH. J. Am. Chem. Soc. 2004;126:11130. Van Veldhuizen JJ, Campbell JE, Giudici RE, Hoveyda AH. J. Am. Chem. Soc. 2005;127:6877. Kacprzynski MA, May TL, Kazane SA, Hoveyda AH. Angew. Chem., Int. Ed. 2007;46:4554.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

