Syndecan-1 antigen, a promising new target for triple-negative breast cancer immuno-PET and radioimmunotherapy. A preclinical study on MDA-MB-468 xenograft tumors
- PMID: 22214534
- PMCID: PMC3250983
- DOI: 10.1186/2191-219X-1-20
Syndecan-1 antigen, a promising new target for triple-negative breast cancer immuno-PET and radioimmunotherapy. A preclinical study on MDA-MB-468 xenograft tumors
Abstract
Background: Overexpression of syndecan-1 (CD138) in breast carcinoma correlates with a poor prognosis and an aggressive phenotype. The objective of this study was to evaluate the potential of targeting CD138 by immuno-PET imaging and radioimmunotherapy (RIT) using the antihuman syndecan-1 B-B4 mAb radiolabeled with either 124I or 131I in nude mice engrafted with the triple-negative MDA-MB-468 breast cancer cell line.
Method: The immunoreactivity of 125I-B-B4 (80%) was determined, and the affinity of 125I-B-B4 and the expression of CD138 on MDA-MB-468 was measured in vitro by Scatchard analysis. CD138 expression on established tumors was confirmed by immunohistochemistry. A biodistribution study was performed in mice with subcutaneous MDA-MB-468 and 125I-B-B4 at 4, 24, 48, 72, and 96 h after injection and compared with an isotype-matched control. Tumor uptake of B-B4 was evaluated in vivo by immuno-PET imaging using the 124I-B-B4 mAb. The maximum tolerated dose (MTD) was determined from mice treated with 131I-B-B4 and the RIT efficacy evaluated.
Results: 125I-B-B4 affinity was in the nanomolar range (Kd = 4.39 ± 1.10 nM). CD138 expression on MDA-MB-468 cells was quite low (Bmax = 1.19 × 104 ± 9.27 × 102 epitopes/cell) but all expressed CD138 in vivo as determined by immunohistochemistry. The tumor uptake of 125I-B-B4 peaked at 14% injected dose (ID) per gram at 24 h and was higher than that of the isotype-matched control mAb (5% ID per gram at 24 h). Immuno-PET performed with 124I-B-B4 on tumor-bearing mice confirmed the specificity of B-B4 uptake and its retention within the tumor. The MTD was reached at 22.2 MBq. All mice treated with RIT (n = 8) as a single treatment at the MTD experienced a partial (n = 3) or complete (n = 5) response, with three of them remaining tumor-free 95 days after treatment.
Conclusion: These results demonstrate that RIT with 131I-B-B4 could be considered for the treatment of metastatic triple-negative breast cancer that cannot benefit from hormone therapy or anti-Her2/neu immunotherapy. Immuno-PET for visualizing CD138-expressing tumors with 124I-B-B4 reinforces the interest of this mAb for diagnosis and quantitative imaging.
Figures
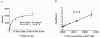




Similar articles
-
Radioimmunotherapy of head and neck cancer xenografts using 131I-labeled antibody L19-SIP for selective targeting of tumor vasculature.J Nucl Med. 2006 Jul;47(7):1127-35. J Nucl Med. 2006. PMID: 16818947
-
Panitumumab-DOTA-111In: An Epidermal Growth Factor Receptor Targeted Theranostic for SPECT/CT Imaging and Meitner-Auger Electron Radioimmunotherapy of Triple-Negative Breast Cancer.Mol Pharm. 2022 Oct 3;19(10):3652-3663. doi: 10.1021/acs.molpharmaceut.2c00457. Epub 2022 Aug 4. Mol Pharm. 2022. PMID: 35926098
-
High-quality 124I-labelled monoclonal antibodies for use as PET scouting agents prior to 131I-radioimmunotherapy.Eur J Nucl Med Mol Imaging. 2004 Dec;31(12):1645-52. doi: 10.1007/s00259-004-1632-8. Epub 2004 Jul 31. Eur J Nucl Med Mol Imaging. 2004. PMID: 15290121
-
Dosimetry results suggest feasibility of radioimmunotherapy using anti-CD138 (B-B4) antibody in multiple myeloma patients.Tumour Biol. 2012 Jun;33(3):679-88. doi: 10.1007/s13277-012-0362-y. Epub 2012 Mar 3. Tumour Biol. 2012. PMID: 22389160
-
Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials?Front Pharmacol. 2016 May 24;7:131. doi: 10.3389/fphar.2016.00131. eCollection 2016. Front Pharmacol. 2016. PMID: 27252651 Free PMC article. Review.
Cited by
-
Prognostic impact of tumour-associated B cells and plasma cells in oesophageal and gastric adenocarcinoma.J Gastrointest Oncol. 2016 Dec;7(6):848-859. doi: 10.21037/jgo.2016.11.07. J Gastrointest Oncol. 2016. PMID: 28078109 Free PMC article.
-
Nucleic acid-triggered tumoral immunity propagates pH-selective therapeutic antibodies through tumor-driven epitope spreading.Cancer Sci. 2023 Jan;114(1):321-338. doi: 10.1111/cas.15596. Epub 2022 Oct 17. Cancer Sci. 2023. PMID: 36136061 Free PMC article.
-
Revisiting the Syndecans: Master Signaling Regulators with Prognostic and Targetable Therapeutic Values in Breast Carcinoma.Cancers (Basel). 2023 Mar 16;15(6):1794. doi: 10.3390/cancers15061794. Cancers (Basel). 2023. PMID: 36980680 Free PMC article. Review.
-
Triple Negative Breast Cancer: Nanosolutions for a Big Challenge.Adv Sci (Weinh). 2015 Jul 17;2(11):1500053. doi: 10.1002/advs.201500053. eCollection 2015 Nov. Adv Sci (Weinh). 2015. PMID: 27980912 Free PMC article.
-
The role of syndecan-1 in cellular signaling and its effects on heparan sulfate biosynthesis in mesenchymal tumors.Front Oncol. 2013 Dec 19;3:310. doi: 10.3389/fonc.2013.00310. Front Oncol. 2013. PMID: 24392351 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

