Imaging technologies for preclinical models of bone and joint disorders
- PMID: 22214535
- PMCID: PMC3251252
- DOI: 10.1186/2191-219X-1-11
Imaging technologies for preclinical models of bone and joint disorders
Abstract
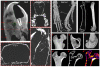
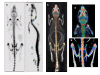
Preclinical models for musculoskeletal disorders are critical for understanding the pathogenesis of bone and joint disorders in humans and the development of effective therapies. The assessment of these models primarily relies on morphological analysis which remains time consuming and costly, requiring large numbers of animals to be tested through different stages of the disease. The implementation of preclinical imaging represents a keystone in the refinement of animal models allowing longitudinal studies and enabling a powerful, non-invasive and clinically translatable way for monitoring disease progression in real time. Our aim is to highlight examples that demonstrate the advantages and limitations of different imaging modalities including magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), single-photon emission computed tomography (SPECT) and optical imaging. All of which are in current use in preclinical skeletal research. MRI can provide high resolution of soft tissue structures, but imaging requires comparatively long acquisition times; hence, animals require long-term anaesthesia. CT is extensively used in bone and joint disorders providing excellent spatial resolution and good contrast for bone imaging. Despite its excellent structural assessment of mineralized structures, CT does not provide in vivo functional information of ongoing biological processes. Nuclear medicine is a very promising tool for investigating functional and molecular processes in vivo with new tracers becoming available as biomarkers. The combined use of imaging modalities also holds significant potential for the assessment of disease pathogenesis in animal models of musculoskeletal disorders, minimising the use of conventional invasive methods and animal redundancy.
Figures




References
-
- European Science Foundation. Rheumatic Disease-a Major Challenge for European Research and Health Care. European Science Foundation Policy Briefing. 2006;6:1–4.
-
- Vanderheyden JL. The use of imaging in preclinical drug development. Q J Nucl Med Mol Imaging. 2009;53:374–381. - PubMed
-
- Holdsworth DW, Thornton M. Micro-CT in small animal and specimen imaging. Trends in Biotechnology. 2002;20:34–39. doi: 10.1016/S0167-7799(02)02004-8. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

