Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis
- PMID: 22214769
- PMCID: PMC3249626
- DOI: 10.1101/cshperspect.a008227
Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis
Abstract
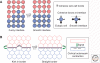
The establishment and maintenance of precisely organized tissues requires the formation of sharp borders between distinct cell populations. The maintenance of segregated cell populations is also required for tissue homeostasis in the adult, and deficiencies in segregation underlie the metastatic spreading of tumor cells. Three classes of mechanisms that underlie cell segregation and border formation have been uncovered. The first involves differences in cadherin-mediated cell-cell adhesion that establishes interfacial tension at the border between distinct cell populations. A second mechanism involves the induction of actomyosin-mediated contraction by intercellular signaling, such that cortical tension is generated at the border. Third, activation of Eph receptors and ephrins can lead to both decreased adhesion by triggering cleavage of E-cadherin, and to repulsion of cells by regulation of the actin cytoskeleton, thus preventing intermingling between cell populations. These mechanisms play crucial roles at distinct boundaries during development, and alterations in cadherin or Eph/ephrin expression have been implicated in tumor metastasis.
Figures


References
-
- Abercrombie M 1979. Contact inhibition and malignancy. Nature 281: 259–262 - PubMed
-
- Abercrombie M, Heaysman JE 1953. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res 5: 111–131 - PubMed
-
- Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD 2010. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol 12: 1194–1204 - PubMed
-
- Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW 2003. Eph/ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol 13: 1571–1582 - PubMed
-
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. 2002. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
