The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis
- PMID: 22214817
- PMCID: PMC3291248
- DOI: 10.1104/pp.111.189167
The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis
Abstract
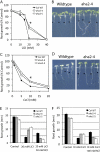
The plasma membrane proton gradient is an essential feature of plant cells. In Arabidopsis (Arabidopsis thaliana), this gradient is generated by the plasma membrane proton pump encoded by a family of 11 genes (abbreviated as AHA, for Arabidopsis H(+)-ATPase), of which AHA1 and AHA2 are the two most predominantly expressed in seedlings and adult plants. Although double knockdown mutant plants containing T-DNA insertions in both genes are embryonic lethal, under ideal laboratory growth conditions, single knockdown mutant plants with a 50% reduction in proton pump concentration complete their life cycle without any observable growth alteration. However, when grown under conditions that induce stress on the plasma membrane protonmotive force (PMF), such as high external potassium to reduce the electrical gradient or high external pH to reduce the proton chemical gradient, aha2 mutant plants show a growth retardation compared with wild-type plants. In this report, we describe the results of studies that examine in greater detail AHA2's specific role in maintaining the PMF during seedling growth. By comparing the wild type and aha2 mutants, we have measured the effects of a reduced PMF on root and hypocotyl growth, ATP-induced skewed root growth, and rapid cytoplasmic calcium spiking. In addition, genome-wide gene expression profiling revealed the up-regulation of potassium transporters in aha2 mutants, indicating, as predicted, a close link between the PMF and potassium uptake at the plasma membrane. Overall, this characterization of aha2 mutants provides an experimental and theoretical framework for investigating growth and signaling processes that are mediated by PMF-coupled energetics at the cell membrane.
Figures




Similar articles
-
Roles of plasma membrane proton ATPases AHA2 and AHA7 in normal growth of roots and root hairs in Arabidopsis thaliana.Physiol Plant. 2019 Jul;166(3):848-861. doi: 10.1111/ppl.12842. Epub 2018 Nov 20. Physiol Plant. 2019. PMID: 30238999 Free PMC article.
-
Environmental and Genetic Factors Regulating Localization of the Plant Plasma Membrane H+-ATPase.Plant Physiol. 2018 Jan;176(1):364-377. doi: 10.1104/pp.17.01126. Epub 2017 Oct 17. Plant Physiol. 2018. PMID: 29042459 Free PMC article.
-
Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity.J Biol Chem. 2010 Jun 4;285(23):17918-29. doi: 10.1074/jbc.M110.101733. Epub 2010 Mar 26. J Biol Chem. 2010. PMID: 20348108 Free PMC article.
-
Regulation of the plasma membrane proton pump (H(+)-ATPase) by phosphorylation.Curr Opin Plant Biol. 2015 Dec;28:68-75. doi: 10.1016/j.pbi.2015.09.005. Epub 2015 Oct 24. Curr Opin Plant Biol. 2015. PMID: 26476298 Free PMC article. Review.
-
Animal plasma membrane energization by chemiosmotic H+ V-ATPases.J Exp Biol. 1997 Jan;200(Pt 2):203-16. doi: 10.1242/jeb.200.2.203. J Exp Biol. 1997. PMID: 9050228 Review.
Cited by
-
Lipid kinases PIP5K7 and PIP5K9 are required for polyamine-triggered K+ efflux in Arabidopsis roots.Plant J. 2020 Oct;104(2):416-432. doi: 10.1111/tpj.14932. Epub 2020 Aug 19. Plant J. 2020. PMID: 32666545 Free PMC article.
-
Uncovering pH at both sides of the root plasma membrane interface using noninvasive imaging.Proc Natl Acad Sci U S A. 2018 Jun 19;115(25):6488-6493. doi: 10.1073/pnas.1721769115. Epub 2018 Jun 4. Proc Natl Acad Sci U S A. 2018. PMID: 29866831 Free PMC article.
-
Integrated regulation triggered by a cryophyte ω-3 desaturase gene confers multiple-stress tolerance in tobacco.J Exp Bot. 2018 Apr 9;69(8):2131-2148. doi: 10.1093/jxb/ery050. J Exp Bot. 2018. PMID: 29432580 Free PMC article.
-
Phosphoproteomic Analyses Reveal Early Signaling Events in the Osmotic Stress Response.Plant Physiol. 2014 Jul;165(3):1171-1187. doi: 10.1104/pp.114.238816. Epub 2014 May 7. Plant Physiol. 2014. PMID: 24808101 Free PMC article.
-
Constitutive Expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in Tomato Confers Auxin-Independent Hypocotyl Elongation.Plant Physiol. 2017 Feb;173(2):1453-1462. doi: 10.1104/pp.16.01514. Epub 2016 Dec 20. Plant Physiol. 2017. PMID: 27999086 Free PMC article.
References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. (2004) Image processing with ImageJ. Biophotonics Int 11: 36–42
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

