Neural stem cell depletion and CNS developmental defects after enteroviral infection
- PMID: 22214838
- PMCID: PMC3349883
- DOI: 10.1016/j.ajpath.2011.11.016
Neural stem cell depletion and CNS developmental defects after enteroviral infection
Abstract
Coxsackieviruses are significant human pathogens causing myocarditis, meningitis, and encephalitis. We previously demonstrated the ability of coxsackievirus B3 (CVB3) to persist within the neonatal central nervous system (CNS) and to target neural stem cells. Given that CVB3 is a cytolytic virus and may therefore damage target cells, we characterized the potential reduction in neurogenesis within the developing brain and the subsequent developmental defects that occurred after the loss of these essential neural stem cells. Neonatal mice were inoculated with a recombinant CVB3 expressing eGFP (eGFP-CVB3), and alterations in neurogenesis and brain development were evaluated over time. We observed a reduction in proliferating cells in CNS neurogenic regions simultaneously with the presence of nestin(+) cells undergoing apoptosis. The size of the brain appeared smaller by histology, and a permanent decrease in brain wet weight was observed after eGFP-CVB3 infection. We also observed an inverse relationship between the amount of virus material and brain wet weight up to day 30 postinfection. In addition, signs of astrogliosis and a compaction of the cortical layers were observed at 90 days postinfection. Intriguingly, partial brain wet weight recovery was observed in mice treated with the antiviral drug ribavirin during the persistent stage of infection. Hence, long-term neurological sequelae might be expected after neonatal enteroviral infections, yet antiviral treatment initiated long after the end of acute infection might limit virus-mediated neuropathology.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


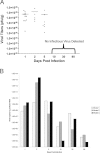


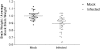


Similar articles
-
Coxsackievirus preferentially replicates and induces cytopathic effects in undifferentiated neural progenitor cells.J Virol. 2011 Jun;85(12):5718-32. doi: 10.1128/JVI.02261-10. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471247 Free PMC article.
-
Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease.Am J Pathol. 2003 Oct;163(4):1379-93. doi: 10.1016/S0002-9440(10)63496-7. Am J Pathol. 2003. PMID: 14507646 Free PMC article.
-
Coxsackievirus targets proliferating neuronal progenitor cells in the neonatal CNS.J Neurosci. 2005 Mar 2;25(9):2434-44. doi: 10.1523/JNEUROSCI.4517-04.2005. J Neurosci. 2005. PMID: 15745971 Free PMC article.
-
Viral persistence and chronic immunopathology in the adult central nervous system following Coxsackievirus infection during the neonatal period.J Virol. 2009 Sep;83(18):9356-69. doi: 10.1128/JVI.02382-07. Epub 2009 Jul 1. J Virol. 2009. PMID: 19570873 Free PMC article.
-
Type B coxsackieviruses and central nervous system disorders: critical review of reported associations.Rev Med Virol. 2021 Jul;31(4):e2191. doi: 10.1002/rmv.2191. Epub 2020 Nov 7. Rev Med Virol. 2021. PMID: 33159700 Review.
Cited by
-
Coxsackievirus B3 infects the bone marrow and diminishes the restorative capacity of erythroid and lymphoid progenitors.J Virol. 2013 Mar;87(5):2823-34. doi: 10.1128/JVI.03004-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269810 Free PMC article.
-
Sublethal enteroviral infection exacerbates disease progression in an ALS mouse model.J Neuroinflammation. 2022 Jan 12;19(1):16. doi: 10.1186/s12974-022-02380-7. J Neuroinflammation. 2022. PMID: 35022041 Free PMC article.
-
T cell mediated suppression of neurotropic coronavirus replication in neural precursor cells.Virology. 2014 Jan 20;449:235-43. doi: 10.1016/j.virol.2013.11.025. Epub 2013 Dec 12. Virology. 2014. PMID: 24418558 Free PMC article.
-
Neural precursor cells derived from induced pluripotent stem cells exhibit reduced susceptibility to infection with a neurotropic coronavirus.Virology. 2017 Nov;511:49-55. doi: 10.1016/j.virol.2017.08.003. Epub 2017 Aug 17. Virology. 2017. PMID: 28822268 Free PMC article.
-
Enterovirus and Encephalitis.Front Microbiol. 2020 Feb 20;11:261. doi: 10.3389/fmicb.2020.00261. eCollection 2020. Front Microbiol. 2020. PMID: 32153545 Free PMC article. Review.
References
-
- Berger J.R., Chumley W., Pittman T., Given C., Nuovo G. Persistent Coxsackie B encephalitis: report of a case and review of the literature. J Neurovirol. 2006;12:511–516. - PubMed
-
- Kamei S., Hersch S.M., Kurata T., Takei Y. Coxsackie B antigen in the central nervous system of a patient with fatal acute encephalitis: immunohistochemical studies of formalin-fixed paraffin-embedded tissue. Acta Neuropathol (Berl) 1990;80:216–221. - PubMed
-
- Whitton J.L., Cornell C.T., Feuer R. Host and virus determinants of picornavirus pathogenesis and tropism. Nat Rev Microbiol. 2005;3:765–776. - PubMed
-
- Romero J.R. Pediatric group B coxsackievirus infections. Curr Top Microbiol Immunol. 2008;323:223–239. - PubMed
-
- Wikswo M.E., Khetsuriani N., Fowlkes A.L., Zheng X., Penaranda S., Verma N., Shulman S.T., Sircar K., Robinson C.C., Schmidt T., Schnurr D., Oberste M.S. Increased activity of Coxsackievirus B1 strains associated with severe disease among young infants in the United States, 2007–2008. Clin Infect Dis. 2009;49:e44–e51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

