Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor
- PMID: 22215085
- PMCID: PMC3272563
- DOI: 10.1038/ncomms1607
Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor
Abstract
Albumin is the most abundant protein in blood where it has a pivotal role as a transporter of fatty acids and drugs. Like IgG, albumin has long serum half-life, protected from degradation by pH-dependent recycling mediated by interaction with the neonatal Fc receptor, FcRn. Although the FcRn interaction with IgG is well characterized at the atomic level, its interaction with albumin is not. Here we present structure-based modelling of the FcRn-albumin complex, supported by binding analysis of site-specific mutants, providing mechanistic evidence for the presence of pH-sensitive ionic networks at the interaction interface. These networks involve conserved histidines in both FcRn and albumin domain III. Histidines also contribute to intramolecular interactions that stabilize the otherwise flexible loops at both the interacting surfaces. Molecular details of the FcRn-albumin complex may guide the development of novel albumin variants with altered serum half-life as carriers of drugs.
Conflict of interest statement
I.S., J.T.A., B.D., J.C., A.P., L.E. and D.S. are co-inventors on pending patent applications, which are entitled 'Albumin Variants' and relate to the data described in this paper. The remaining authors have no competing financial interest to declare.
Figures
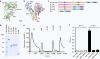
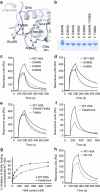

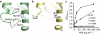
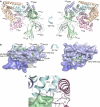
References
-
- Peters T. Jr. Serum albumin. Adv. Protein Chem. 37, 161–245 (1985). - PubMed
-
- Anderson C. L. et al.. Perspective–FcRn transports albumin: relevance to immunology and medicine. Trends Immunol. 27, 343–348 (2006). - PubMed
-
- Roopenian D. C. & Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

