The structures of nonprotein-coding RNAs that drive internal ribosome entry site function
- PMID: 22215521
- PMCID: PMC3973487
- DOI: 10.1002/wrna.1105
The structures of nonprotein-coding RNAs that drive internal ribosome entry site function
Abstract
Internal ribosome entry sites (IRESs) are RNA sequences that can recruit the translation machinery independent of the 5' end of the messenger RNA. IRESs are found in both viral and cellular RNAs and are important for regulating gene expression. There is great diversity in the mechanisms used by IRESs to recruit the ribosome and this is reflected in a variety of RNA sequences that function as IRESs. The ability of an RNA sequence to function as an IRES is conferred by structures operating at multiple levels from primary sequence through higher-order three-dimensional structures within dynamic ribonucleoproteins (RNPs). When these diverse structures are compared, some trends are apparent, but overall it is not possible to find universal rules to describe IRES structure and mechanism. Clearly, many different sequences and structures have evolved to perform the function of recruiting, positioning, and activating a ribosome without using the canonical cap-dependent mechanism. However, as our understanding of the specific sequences, structures, and mechanisms behind IRES function improves, more common features may emerge to link these diverse RNAs.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures

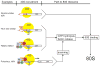
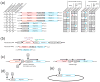
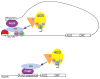
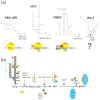


Similar articles
-
A dynamic RNA loop in an IRES affects multiple steps of elongation factor-mediated translation initiation.Elife. 2015 Nov 2;4:e08146. doi: 10.7554/eLife.08146. Elife. 2015. PMID: 26523395 Free PMC article.
-
Structural basis for ribosome recruitment and manipulation by a viral IRES RNA.Science. 2006 Dec 1;314(5804):1450-4. doi: 10.1126/science.1133281. Epub 2006 Nov 23. Science. 2006. PMID: 17124290 Free PMC article.
-
Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites.EMBO Rep. 2001 Oct;2(10):893-8. doi: 10.1093/embo-reports/kve208. EMBO Rep. 2001. PMID: 11600453 Free PMC article. Review.
-
Viral IRES RNA structures and ribosome interactions.Trends Biochem Sci. 2008 Jun;33(6):274-83. doi: 10.1016/j.tibs.2008.04.007. Epub 2008 May 28. Trends Biochem Sci. 2008. PMID: 18468443 Free PMC article. Review.
-
Tricks an IRES uses to enslave ribosomes.Trends Microbiol. 2012 Nov;20(11):558-66. doi: 10.1016/j.tim.2012.08.002. Epub 2012 Aug 31. Trends Microbiol. 2012. PMID: 22944245 Free PMC article. Review.
Cited by
-
Ligand-responsive RNA mechanical switches.RNA Biol. 2015;12(8):780-6. doi: 10.1080/15476286.2015.1054592. RNA Biol. 2015. PMID: 26158858 Free PMC article.
-
Functional analysis of Kaposi's sarcoma-associated herpesvirus vFLIP expression reveals a new mode of IRES-mediated translation.RNA. 2014 Nov;20(11):1803-14. doi: 10.1261/rna.045328.114. Epub 2014 Sep 22. RNA. 2014. PMID: 25246653 Free PMC article.
-
Molecular analysis of the factorless internal ribosome entry site in Cricket Paralysis virus infection.Sci Rep. 2016 Nov 17;6:37319. doi: 10.1038/srep37319. Sci Rep. 2016. PMID: 27853311 Free PMC article.
-
A dynamic RNA loop in an IRES affects multiple steps of elongation factor-mediated translation initiation.Elife. 2015 Nov 2;4:e08146. doi: 10.7554/eLife.08146. Elife. 2015. PMID: 26523395 Free PMC article.
-
Ribosomal protein S25 dependency reveals a common mechanism for diverse internal ribosome entry sites and ribosome shunting.Mol Cell Biol. 2013 Mar;33(5):1016-26. doi: 10.1128/MCB.00879-12. Epub 2012 Dec 28. Mol Cell Biol. 2013. PMID: 23275440 Free PMC article.
References
-
- Mathews MB, Sonenberg N, Hershey BJW. Origins and Principles of Translational Control. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 1–40.
-
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. - PubMed
-
- Pestova T, Lorsch JR, Hellen CU. The Mechanism of Translation Initiation in Eukaryotes. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 87–128.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

