Daily rhythms in olfactory discrimination depend on clock genes but not the suprachiasmatic nucleus
- PMID: 22215613
- PMCID: PMC3658462
- DOI: 10.1177/0748730411420247
Daily rhythms in olfactory discrimination depend on clock genes but not the suprachiasmatic nucleus
Abstract
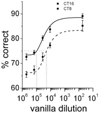
The suprachiasmatic nucleus (SCN) regulates a wide range of daily behaviors and has been described as the master circadian pacemaker. The role of daily rhythmicity in other tissues, however, is unknown. We hypothesized that circadian changes in olfactory discrimination depend on a genetic circadian oscillator outside the SCN. We developed an automated assay to monitor olfactory discrimination in individual mice throughout the day. We found olfactory sensitivity increased approximately 6-fold from a minimum during the day to a peak in the early night. This circadian rhythm was maintained in SCN-lesioned mice and mice deficient for the Npas2 gene but was lost in mice lacking Bmal1 or both Per1 and Per2 genes. We conclude that daily rhythms in olfactory sensitivity depend on the expression of canonical clock genes. Olfaction is, thus, the first circadian behavior that is not based on locomotor activity and does not require the SCN.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
Genetic interaction of Per1 and Dec1/2 in the regulation of circadian locomotor activity.J Biol Rhythms. 2011 Dec;26(6):530-40. doi: 10.1177/0748730411419782. J Biol Rhythms. 2011. PMID: 22215611
-
Advanced light-entrained activity onsets and restored free-running suprachiasmatic nucleus circadian rhythms in per2/dec mutant mice.Chronobiol Int. 2011 Nov;28(9):737-50. doi: 10.3109/07420528.2011.607374. Chronobiol Int. 2011. PMID: 22080784
-
Functional central rhythmicity and light entrainment, but not liver and muscle rhythmicity, are Clock independent.Am J Physiol Regul Integr Comp Physiol. 2006 Oct;291(4):R1172-80. doi: 10.1152/ajpregu.00223.2006. Epub 2006 May 18. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16709646
-
The Retinal Circadian Clock and Photoreceptor Viability.Adv Exp Med Biol. 2018;1074:345-350. doi: 10.1007/978-3-319-75402-4_42. Adv Exp Med Biol. 2018. PMID: 29721962 Free PMC article. Review.
-
[Synchronization and genetic redundancy in circadian clocks].Med Sci (Paris). 2008 Mar;24(3):270-6. doi: 10.1051/medsci/2008243270. Med Sci (Paris). 2008. PMID: 18334175 Review. French.
Cited by
-
Impact of Targeted Deletion of the Circadian Clock Gene Bmal1 in Excitatory Forebrain Neurons on Adult Neurogenesis and Olfactory Function.Int J Mol Sci. 2020 Feb 19;21(4):1394. doi: 10.3390/ijms21041394. Int J Mol Sci. 2020. PMID: 32092990 Free PMC article.
-
Molecular clock regulates daily α1-2-fucosylation of the neural cell adhesion molecule (NCAM) within mouse secondary olfactory neurons.J Biol Chem. 2014 Dec 26;289(52):36158-65. doi: 10.1074/jbc.M114.571141. Epub 2014 Nov 10. J Biol Chem. 2014. PMID: 25384980 Free PMC article.
-
Potential neural substrates underlying circadian and olfactory disruptions in preclinical Alzheimer's disease.Front Neurosci. 2023 Nov 29;17:1295998. doi: 10.3389/fnins.2023.1295998. eCollection 2023. Front Neurosci. 2023. PMID: 38094003 Free PMC article. Review.
-
Cardinal Epigenetic Role of non-coding Regulatory RNAs in Circadian Rhythm.Mol Neurobiol. 2018 Apr;55(4):3564-3576. doi: 10.1007/s12035-017-0573-8. Epub 2017 May 17. Mol Neurobiol. 2018. PMID: 28516429 Review.
-
Circadian rhythm mechanism in the suprachiasmatic nucleus and its relation to the olfactory system.Front Neural Circuits. 2024 Mar 25;18:1385908. doi: 10.3389/fncir.2024.1385908. eCollection 2024. Front Neural Circuits. 2024. PMID: 38590628 Free PMC article. Review.
References
-
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J. In rats, odor-induced Fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci Lett. 1999;272:175–178. - PubMed
-
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential Functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

