Interleukin 33 as a mechanically responsive cytokine secreted by living cells
- PMID: 22215666
- PMCID: PMC3307313
- DOI: 10.1074/jbc.M111.298703
Interleukin 33 as a mechanically responsive cytokine secreted by living cells
Abstract
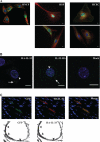
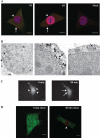
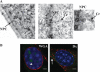
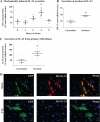
Interleukin 33 (IL-33), a member of the Interleukin 1 cytokine family, is implicated in numerous human inflammatory diseases such as asthma, atherosclerosis, and rheumatoid arthritis. Despite its pathophysiologic importance, fundamental questions regarding the basic biology of IL-33 remain. Nuclear localization and lack of an export signal sequence are consistent with the view of IL-33 as a nuclear factor with the ability to repress RNA transcription. However, signaling via the transmembrane receptor ST2 and documented caspase-dependent inactivation have suggested IL-33 is liberated during cellular necrosis to effect paracrine signaling. We determined the subcellular localization of IL-33 and tracked its intracellular mobility and extracellular release. In contrast to published data, IL-33 localized simultaneously to nuclear euchromatin and membrane-bound cytoplasmic vesicles. Fluorescent pulse-chase fate-tracking documented dynamic nucleo-cytoplasmic flux, which was dependent on nuclear pore complex function. In murine fibroblasts in vitro and in vivo, mechanical strain induced IL-33 secretion in the absence of cellular necrosis. These data document IL-33 dynamic inter-organelle trafficking and release during biomechanical overload. As such we recharacterize IL-33 as both an inflammatory as well as mechanically responsive cytokine secreted by living cells.
Figures

References
-
- Haraldsen G., Balogh J., Pollheimer J., Sponheim J., Küchler A. M. (2009) Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 30, 227–233 - PubMed
-
- Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

