AMP is an adenosine A1 receptor agonist
- PMID: 22215671
- PMCID: PMC3285310
- DOI: 10.1074/jbc.M111.291666
AMP is an adenosine A1 receptor agonist
Abstract
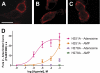
Numerous receptors for ATP, ADP, and adenosine exist; however, it is currently unknown whether a receptor for the related nucleotide adenosine 5'-monophosphate (AMP) exists. Using a novel cell-based assay to visualize adenosine receptor activation in real time, we found that AMP and a non-hydrolyzable AMP analog (deoxyadenosine 5'-monophosphonate, ACP) directly activated the adenosine A(1) receptor (A(1)R). In contrast, AMP only activated the adenosine A(2B) receptor (A(2B)R) after hydrolysis to adenosine by ecto-5'-nucleotidase (NT5E, CD73) or prostatic acid phosphatase (PAP, ACPP). Adenosine and AMP were equipotent human A(1)R agonists in our real-time assay and in a cAMP accumulation assay. ACP also depressed cAMP levels in mouse cortical neurons through activation of endogenous A(1)R. Non-selective purinergic receptor antagonists (pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid and suramin) did not block adenosine- or AMP-evoked activation. Moreover, mutation of His-251 in the human A(1)R ligand binding pocket reduced AMP potency without affecting adenosine potency. In contrast, mutation of a different binding pocket residue (His-278) eliminated responses to AMP and to adenosine. Taken together, our study indicates that the physiologically relevant nucleotide AMP is a full agonist of A(1)R. In addition, our study suggests that some of the physiological effects of AMP may be direct, and not indirect through ectonucleotidases that hydrolyze this nucleotide to adenosine.
Figures


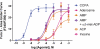


References
-
- Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 - PubMed
-
- Inbe H., Watanabe S., Miyawaki M., Tanabe E., Encinas J. A. (2004) Identification and characterization of a cell surface receptor, P2Y15, for AMP and adenosine. J. Biol. Chem. 279, 19790–19799 - PubMed
-
- Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Miras-Portugal M. T., King B. F., Gachet C., Jacobson K. A., Weisman G. A. (2005) The recently deorphanized GPR80 (GPR99) proposed to be the P2Y15 receptor is not a genuine P2Y receptor. Trends Pharmacol. Sci. 26, 8–9 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

