Ringo/cyclin-dependent kinase and mitogen-activated protein kinase signaling pathways regulate the activity of the cell fate determinant Musashi to promote cell cycle re-entry in Xenopus oocytes
- PMID: 22215682
- PMCID: PMC3323046
- DOI: 10.1074/jbc.M111.300681
Ringo/cyclin-dependent kinase and mitogen-activated protein kinase signaling pathways regulate the activity of the cell fate determinant Musashi to promote cell cycle re-entry in Xenopus oocytes
Abstract
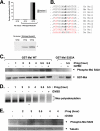
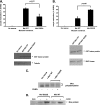
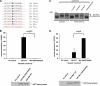
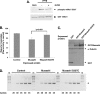
Cell cycle re-entry during vertebrate oocyte maturation is mediated through translational activation of select target mRNAs, culminating in the activation of mitogen-activated protein kinase and cyclin B/cyclin-dependent kinase (CDK) signaling. The temporal order of targeted mRNA translation is crucial for cell cycle progression and is determined by the timing of activation of distinct mRNA-binding proteins. We have previously shown in oocytes from Xenopus laevis that the mRNA-binding protein Musashi targets translational activation of early class mRNAs including the mRNA encoding the Mos proto-oncogene. However, the molecular mechanism by which Musashi function is activated is unknown. We report here that activation of Musashi1 is mediated by Ringo/CDK signaling, revealing a novel role for early Ringo/CDK function. Interestingly, Musashi1 activation is subsequently sustained through mitogen-activated protein kinase signaling, the downstream effector of Mos mRNA translation, thus establishing a positive feedback loop to amplify Musashi function. The identified regulatory sites are present in mammalian Musashi proteins, and our data suggest that phosphorylation may represent an evolutionarily conserved mechanism to control Musashi-dependent target mRNA translation.
Figures


References
-
- Bettegowda A., Smith G. W. (2007) Mechanisms of maternal mRNA regulation. Implications for mammalian early embryonic development. Front. Biosci. 12, 3713–3726 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

