Hsp90 molecular chaperone inhibitors: are we there yet?
- PMID: 22215907
- PMCID: PMC3252205
- DOI: 10.1158/1078-0432.CCR-11-1000
Hsp90 molecular chaperone inhibitors: are we there yet?
Abstract
Heat shock protein (Hsp) 90 is an ATP-dependent molecular chaperone that is exploited by malignant cells to support activated oncoproteins, including many cancer-associated kinases and transcription factors, and it is essential for oncogenic transformation. Originally viewed with skepticism, Hsp90 inhibitors are now being actively pursued by the pharmaceutical industry, with 17 agents having entered clinical trials. Investigators established Hsp90's druggability using the natural products geldanamycin and radicicol, which mimic the unusual ATP structure adopted in the chaperone's N-terminal nucleotide-binding pocket and cause potent and selective blockade of ATP binding/hydrolysis, inhibit chaperone function, deplete oncogenic clients, and show antitumor activity. Preclinical data obtained with these natural products have heightened interest in Hsp90 as a drug target, and 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) has shown clinical activity (as defined by Response Evaluation Criteria in Solid Tumors) in HER2+ breast cancer. Many optimized synthetic, small-molecule Hsp90 inhibitors from diverse chemotypes are now in clinical trials. Here, we review the discovery and development of Hsp90 inhibitors and assess their potential. There has been significant learning from studies of the basic biology of Hsp90, as well as translational drug development involving this chaperone, enhanced by the use of Hsp90 inhibitors as chemical probes. Success will likely lie in treating cancers that are addicted to particular driver oncogene products (e.g., HER2, ALK, EGFR, and BRAF) that are sensitive Hsp90 clients, as well as malignancies (especially multiple myeloma) in which buffering of proteotoxic stress is critical for survival. We discuss approaches for enhancing the effectiveness of Hsp90 inhibitors and highlight new chaperone and stress-response pathway targets, including HSF1 and Hsp70.
© 2012 AACR.
Figures




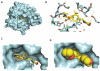
A view of the surface of the entire human N-terminal domain of Hsp90α (in blue) with the resorcylic isoxazole inhibitor NVP-AUY922 (see Figure 2 for chemical structure) bound in the deep ATP pocket.
A more detailed view of NVP-AUY922 in the ATP pocket. The same core network of hydrogen bonding interactions that are exploited by ATP/ADP, geldanamycin and radicicol, including water-mediated ones, are used to anchor this drug and other new synthetic inhibitors into the base of the N-terminal nucleotide-binding pocket. The core structure of the drug is shown in yellow and protein residues are in green. Hydrogen bonding interactions are shown as dotted yellow lines, some of which involve water molecules shown as red spheres. In this view the resorcinol ring is on the left-hand side pointing deep into the base of the pocket, and the two resorcinol hydroxyls groups make key hydrogen bond interactions. The isoxazole ring and morpholine ring (the later on the far right) are viewed side on. The amide substitution on the isoxazole ring extends out of the plane towards the viewer. All of these make additional hydrogen-bonding interactions in the ATP pocket.
The position of the drug is the same as in (b) but the hydrogen bonding interactions have been removed and the protein surface is included, colored light blue. The way the resorcinol ring (on the left, with the two hydroxyl group oxygen atoms colored red) binds deep into the base of the nucleotide binding pocket is clearly visible, and the isopropyl group (on the resorcinol ring and pointing to the right) can be seen binding in a shallow hydrophobic pocket. It can also be seen that the morpholine ring points out of the solvent channel on the right-hand side. Again, the isoxazole ring amide projects out of the plane. Also clear in this representation is that the NVP-AUY922 molecule does not bind in the flat orientation that is depicted in the chemical structure representation (Figure 2). Rather, it adopts a twisted conformation which fits the unusual topology of the binding pocket. These features are generally characteristic of Hsp90 N-terminal inhibitors and they combine to give high levels of potency and selectivity for the target.
The solvent accessible surface of the drug is shown to illustrate how effectively the drug fills the nucleotide binding pocket.

References
-
- Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–53. - PubMed
-
- Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–16. - PubMed
-
- Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, et al. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67:2932–7. - PubMed
-
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

