Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients
- PMID: 22215943
- PMCID: PMC3243885
- DOI: 10.3748/wjg.v17.i47.5184
Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients
Abstract
Aim: To determine whether adding vitamin D, a potent immunomodulator, improves the hepatitis C virus (HCV) response to antiviral therapy.
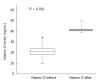
Methods: Seventy-two consecutive patients with chronic HCV genotype 1 were randomized into two groups: the treatment group (n = 36, 50% male, mean age 47 ± 11 years) received Peg-α-2b interferon (1.5 μg/kg per week) plus ribavirin (1000-1200 mg/d) together with vitamin D3 (2000 IU/d, target serum level > 32 ng/mL), and the control group (n = 36, 60% male, mean age 49 ± 7 years) received identical therapy without vitamin D. HCV-RNA was assessed by real-time polymerase chain reaction (sensitivity, 10 IU/mL). The sustained virologic response (SVR) was defined as undetectable HCV-RNA at 24 wk post-treatment.
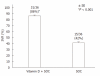
Results: Clinical characteristics were similar in both groups. The treatment group had a higher mean body mass index (27 ± 4 kg/m² vs 24 ± 3 kg/m²; P < 0.01), viral load (50% vs 42%, P < 0.01), and fibrosis score (> F2: 42% vs 19%, P < 0.001) than the controls. At week 4, 16 (44%) treated patients and 6 (17%) controls were HCV-RNA negative (P < 0.001). At week 12, 34 (94%) treated patients and 17 (48%) controls were HCV-RNA negative (P < 0.001). At 24 wk post-treatment (SVR), 31 (86%) treated patients and 15 (42%) controls were HCV-RNA negative (P < 0.001). Viral load, advanced fibrosis and vitamin D supplementation were strongly and independently associated with SVR (multivariate analysis). Adverse events were mild and typical of Peg-α-2b/ribavirin.
Conclusion: Adding vitamin D to conventional Peg-α-2b/ribavirin therapy for treatment-naïve patients with chronic HCV genotype 1 infection significantly improves the viral response.
Keywords: Fibrosis; Genotype 1; Hepatitis C; Sustained viral response; Vitamin D.
Figures

References
-
- Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35–47. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. - PubMed
-
- Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132:1979–1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

