Ror2 enhances polarity and directional migration of primordial germ cells
- PMID: 22216013
- PMCID: PMC3245308
- DOI: 10.1371/journal.pgen.1002428
Ror2 enhances polarity and directional migration of primordial germ cells
Abstract
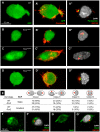
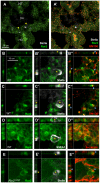
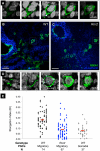
The trafficking of primordial germ cells (PGCs) across multiple embryonic structures to the nascent gonads ensures the transmission of genetic information to the next generation through the gametes, yet our understanding of the mechanisms underlying PGC migration remains incomplete. Here we identify a role for the receptor tyrosine kinase-like protein Ror2 in PGC development. In a Ror2 mouse mutant we isolated in a genetic screen, PGC migration and survival are dysregulated, resulting in a diminished number of PGCs in the embryonic gonad. A similar phenotype in Wnt5a mutants suggests that Wnt5a acts as a ligand to Ror2 in PGCs, although we do not find evidence that WNT5A functions as a PGC chemoattractant. We show that cultured PGCs undergo polarization, elongation, and reorientation in response to the chemotactic factor SCF (secreted KitL), whereas Ror2 PGCs are deficient in these SCF-induced responses. In the embryo, migratory PGCs exhibit a similar elongated geometry, whereas their counterparts in Ror2 mutants are round. The protein distribution of ROR2 within PGCs is asymmetric, both in vitro and in vivo; however, this asymmetry is lost in Ror2 mutants. Together these results indicate that Ror2 acts autonomously to permit the polarized response of PGCs to KitL. We propose a model by which Wnt5a potentiates PGC chemotaxis toward secreted KitL by redistribution of Ror2 within the cell.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
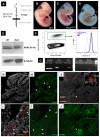
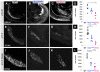
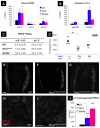
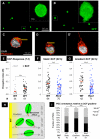
Similar articles
-
Discrete somatic niches coordinate proliferation and migration of primordial germ cells via Wnt signaling.J Cell Biol. 2016 Jul 18;214(2):215-29. doi: 10.1083/jcb.201511061. Epub 2016 Jul 11. J Cell Biol. 2016. PMID: 27402951 Free PMC article.
-
Novel domains of expression for orphan receptor tyrosine kinase Ror2 in the human and mouse reproductive system.Dev Dyn. 2014 Aug;243(8):1037-45. doi: 10.1002/dvdy.24138. Epub 2014 May 6. Dev Dyn. 2014. PMID: 24753105 Free PMC article.
-
Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis.Nat Med. 2012 Feb 19;18(3):405-12. doi: 10.1038/nm.2653. Nat Med. 2012. PMID: 22344299
-
Insight into the role of Wnt5a-induced signaling in normal and cancer cells.Int Rev Cell Mol Biol. 2015;314:117-48. doi: 10.1016/bs.ircmb.2014.10.003. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619716 Review.
-
Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases.Dev Dyn. 2010 Jan;239(1):1-15. doi: 10.1002/dvdy.21991. Dev Dyn. 2010. PMID: 19530173 Review.
Cited by
-
Ror2 as a therapeutic target in cancer.Pharmacol Ther. 2015 Jun;150:143-8. doi: 10.1016/j.pharmthera.2015.01.010. Epub 2015 Jan 19. Pharmacol Ther. 2015. PMID: 25614331 Free PMC article. Review.
-
Building the mammalian testis: origins, differentiation, and assembly of the component cell populations.Genes Dev. 2013 Nov 15;27(22):2409-26. doi: 10.1101/gad.228080.113. Genes Dev. 2013. PMID: 24240231 Free PMC article. Review.
-
Upstream open reading frames control PLK4 translation and centriole duplication in primordial germ cells.Genes Dev. 2022 Jun 1;36(11-12):718-736. doi: 10.1101/gad.349604.122. Epub 2022 Jun 30. Genes Dev. 2022. PMID: 35772791 Free PMC article.
-
Apoptosis in the fetal testis eliminates developmentally defective germ cell clones.Nat Cell Biol. 2020 Dec;22(12):1423-1435. doi: 10.1038/s41556-020-00603-8. Epub 2020 Nov 16. Nat Cell Biol. 2020. PMID: 33199844 Free PMC article.
-
A tug-of-war between germ cell motility and intercellular bridges controls germline cyst formation in mice.Curr Biol. 2024 Dec 16;34(24):5728-5738.e4. doi: 10.1016/j.cub.2024.10.062. Epub 2024 Nov 19. Curr Biol. 2024. PMID: 39566500
References
-
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. - PubMed
-
- Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, et al. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development. 2006;133:4861–4869. - PubMed
-
- Stevens LC. Origin of testicular teratomas from primordial germ cells in mice. J Natl Cancer Inst. 1967;38:549–552. - PubMed
-
- Stevens LC. Germ cell origin of testicular and ovarian teratomas. Transplant Proc. 1984;16:502–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

